TABLE I.
Baseline characteristics of the patients
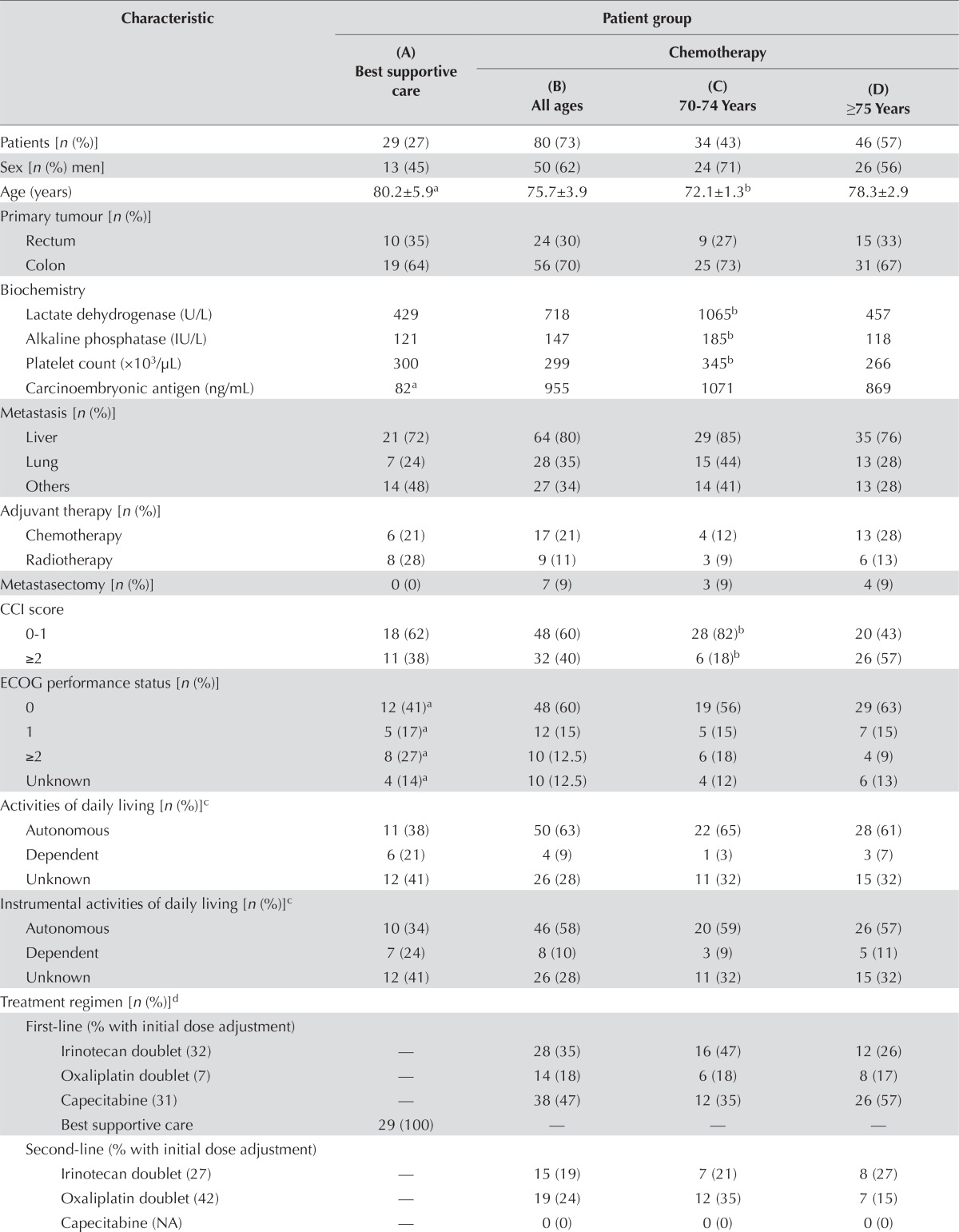
| Characteristic | Patient group | |||
|---|---|---|---|---|
|
| ||||
| (A) Best supportive care | Chemotherapy | |||
|
| ||||
| (B) All ages | (C) 70–74 Years | (D) ≥75 Years | ||
| Patients [n (%)] | 29 (27) | 80 (73) | 34 (43) | 46 (57) |
| Sex [n (%) men] | 13 (45) | 50 (62) | 24 (71) | 26 (56) |
| Age (years) | 80.2±5.9a | 75.7±3.9 | 72.1±1.3b | 78.3±2.9 |
| Primary tumour [n (%)] | ||||
| Rectum | 10 (35) | 24 (30) | 9 (27) | 15 (33) |
| Colon | 19 (64) | 56 (70) | 25 (73) | 31 (67) |
| Biochemistry | ||||
| Lactate dehydrogenase (U/L) | 429 | 718 | 1065b | 457 |
| Alkaline phosphatase (IU/L) | 121 | 147 | 185b | 118 |
| Platelet count (×103/μL) | 300 | 299 | 345b | 266 |
| Carcinoembryonic antigen (ng/mL) | 82a | 955 | 1071 | 869 |
| Metastasis [n (%)] | ||||
| Liver | 21 (72) | 64 (80) | 29 (85) | 35 (76) |
| Lung | 7 (24) | 28 (35) | 15 (44) | 13 (28) |
| Others | 14 (48) | 27 (34) | 14 (41) | 13 (28) |
| Adjuvant therapy [n (%)] | ||||
| Chemotherapy | 6 (21) | 17 (21) | 4 (12) | 13 (28) |
| Radiotherapy | 8 (28) | 9 (11) | 3 (9) | 6 (13) |
| Metastasectomy [n (%)] | 0 (0) | 7 (9) | 3 (9) | 4 (9) |
| CCI score | ||||
| 0–1 | 18 (62) | 48 (60) | 28 (82)b | 20 (43) |
| ≥2 | 11 (38) | 32 (40) | 6 (18)b | 26 (57) |
| ECOG performance status [n (%)] | ||||
| 0 | 12 (41)a | 48 (60) | 19 (56) | 29 (63) |
| 1 | 5 (17)a | 12 (15) | 5 (15) | 7 (15) |
| ≥2 | 8 (27)a | 10 (12.5) | 6 (18) | 4 (9) |
| Unknown | 4 (14)a | 10 (12.5) | 4 (12) | 6 (13) |
| Activities of daily living [n (%)]c | ||||
| Autonomous | 11 (38) | 50 (63) | 22 (65) | 28 (61) |
| Dependent | 6 (21) | 4 (9) | 1 (3) | 3 (7) |
| Unknown | 12 (41) | 26 (28) | 11 (32) | 15 (32) |
| Instrumental activities of daily living [n (%)]c | ||||
| Autonomous | 10 (34) | 46 (58) | 20 (59) | 26 (57) |
| Dependent | 7 (24) | 8 (10) | 3 (9) | 5 (11) |
| Unknown | 12 (41) | 26 (28) | 11 (32) | 15 (32) |
| Treatment regimen [n (%)]d | ||||
| First-line (% with initial dose adjustment) | ||||
| Irinotecan doublet (32) | — | 28 (35) | 16 (47) | 12 (26) |
| Oxaliplatin doublet (7) | — | 14 (18) | 6 (18) | 8 (17) |
| Capecitabine (31) | — | 38 (47) | 12 (35) | 26 (57) |
| Best supportive care | 29 (100) | — | — | — |
| Second-line (% with initial dose adjustment) | ||||
| Irinotecan doublet (27) | — | 15 (19) | 7 (21) | 8 (27) |
| Oxaliplatin doublet (42) | — | 19 (24) | 12 (35) | 7 (15) |
| Capecitabine (NA) | — | 0 (0) | 0 (0) | 0 (0) |
| Third-line (% with initial dose adjustment) | ||||
| Irinotecan doublet (NA) | — | 0 (0) | 0 (0) | 0 (0) |
| Oxaliplatin doublet (43) | — | 7 (9) | 2 (6) | 5 (11) |
| Capecitabine (75) | — | 4 (5) | 4 (12) | 0 (0) |
| Best supportive care | 29 (100) | 69 (86) | 28 (82) | 41 (89) |
| Exposure to all three drugs | — | 28 (35) | 18 (53) | 10 (22) |
| Bevacizumab | ||||
| First-line use | — | 33 (41) | 13 (38) | 20 (43) |
| Second-line use | — | 13 (16) | 8 (24) | 5 (11) |
Significant difference (p ≤ 0.05) compared with (B).
Significant difference (p ≤ 0.05) compared with (D).
Patient groups could not be compared because of missing data.
Statistical tests not applicable.
CCI = Charlson comorbidity index; ECOG = Eastern Cooperative Oncology Group; NA = not applicable.
