Table 1.
First observation of [4+3] cycloadduct 13a.
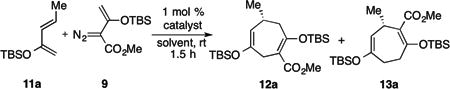
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | solvent | catalyst | ratio[a] 12a : 13a | yield, % | ee of 13a, %[d] |
| 1 | pentane | Rh2(S-PTAD)4 | 94 :6 | 55[b] | -73 |
| 2 | CH2Cl2 | Rh2(S-PTAD)4 | 87 :13 | 43[c] | -71 |
| 3 | pentane | Rh2(S-DOSP)4 | 79 : 21 | 62[c] | 33 |
| 4 | CH2Cl2 | Rh2(S-DOSP)4 | 30 : 70 | 61[c] | 5 |
Determined by 1H NMR of crude reaction mixture,
isolated yield of 12a,
combined yield of 12a and 13a.
negative sign indicates the opposite enantiomer of 13a.
