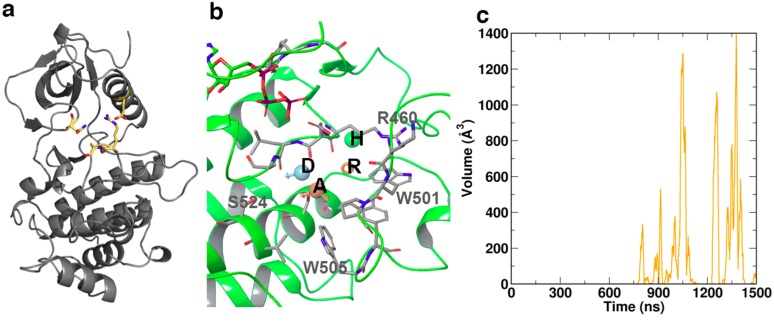
Fig 4. Specific structural features of the various states of ZAP70 kinase domain: (a) The salt bridge D379-R496 and the intermittent hydrogen bonding between N348-S497 (yellow) connect the C- and N-lobes and restrict access to the catalytic cleft.
Additionally these bonds restrict activation loop positioning. (b) The cryptic pocket adjacent to the activation loop spontaneously opened during simulation and was unique to the non-phosphorylated state. Key residues lining the pocket are indicated in gray. Four consensus pharmacophore features were identified by fragment screening. These comprise the acceptor A, donor D, ring R, and a hydrophobic feature H, indicated inset. (c) The cryptic pocket repeatedly opened and closed over the final ~1μs of simulation time, reaching a maximum volume of ~1400 Å 3