Abstract
Long-term treatments with dopaminergic agents are associated with adverse effects, including augmentation. Augmentation consists of an exacerbation of restless legs syndrome (a sleep-related movement disorder) symptoms during treatment compared to those experienced during the period before therapy was initiated. The objective of this study was to examine locomotor activity in rats after long-term dopaminergic treatment and its relationship with expression of the D2 receptor, in addition to demonstrating possible evidence of augmentation. The rats were divided into control (CTRL) and drug (Pramipexole—PPX) groups that received daily saline vehicle and PPX treatments, respectively, for 71 days. The locomotor behavior of the animals was evaluated weekly in the Open Field test for 71 days. The expression of the dopamine D2 receptor was evaluated by Western Blot analysis. The animals that received the PPX demonstrated a significant reduction in locomotor activity from day 1 to day 57 and a significant increase in immobility time from day 1 to day 64 relative to baseline values, but these values had returned to baseline levels at 71 days. No changes in the expression of the D2 receptor were demonstrated after treatment with a dopaminergic agonist. This study suggests changes in locomotor activity in rats after long-term PPX treatment that include an immediate reduction of locomotion and an increase in immobilization, and after 64 days, these values returned to baseline levels without evidence of augmentation. In addition, it was not possible to demonstrate a relationship between locomotor activity and the expression of D2 receptors under these conditions.
Keywords: Augmentation, Dopaminergic agonist, Animal model, Locomotor behavior, Dopaminergic receptor
1. Introduction
Long-term treatment with dopaminergic agents is associated with adverse effects, including daytime sleepiness and sleep attacks, impulse control disorder, addiction, augmentation and other effects [1].
Augmentation occurs in up to 60% of restless legs syndrome (RLS) patients treated with levodopa [2] and, to a lesser extent, dopaminergic agonists [3]. This effect was observed in patients as an increase in severity of RLS symptoms with increasing medication doses. Among the features of augmentation are early onset of symptoms, a decrease in latency to the onset of symptoms the during RLS inactivity, symptoms extending to other body parts (e.g., upper limbs and trunk), and reduced efficacy of medication [4]. There are five known factors that predispose patients to augmentation: drugs with a low half-life, dosage increases, long-term treatments [4], low serum ferritin levels [5], and an RLS-positive family history [6].
Previously, studies on augmentation were not directly based on animal models [5]. The vast majority of the research describes augmentation as a result of the chronic treatment of RLS. Animal models are important tools used to verify hypotheses and decipher the details of pathophysiological mechanisms, including the connections between genes, biology and disease. In this context, considering the effectiveness of PPX on the treatment of RLS and taking into account the results of Chernoloz et al. [7] that demonstrate the facilitatory effect of chronic PPX administration on DA neurotransmission, the assessment of the effect of long-term PPX treatment on locomotor activity in rats has been deemed relevant.
Thus, the objective of this research was to examine locomotor activity in rats after long-term dopaminergic treatment and its relationship with the expression of the D2 receptor and to demonstrate possible evidence of augmentation.
2. Materials and methods
2.1. Animals
A total of 17 three-month-old male Wistar rats (initial and final weight: 270 g and 380 g, respectively) at the Center for Development of Experimental Models for Medicine and Biology (CEDEME-UNIFESP, São Paulo, Brazil) were used in the experiment. The rats were housed in standard polypropylene cages, maintained in a temperature-controlled room (23±1 °C) with a 12:12-h light–dark cycle (lights on at 7 a.m.) and had access to food and water ad libitum. The experimental protocol was approved by the Ethics Committee of UNIFESP (CEP: 0881/11). The animals were divided into control group (n=8) and drug groups (n=9).
2.2. Experimental design
Initially, the animals underwent adaptation to the Open Field (10 min). After the adaptation period and baseline test, treatment was initiated, and the animals received a daily dose of either the drug or saline solution at the same time each day for 71 days. Tests were conducted in the Open Field on days 1, 8, 15, 22, 29, 36, 43, 50, 57, 64, and 71 of treatment. The treatment occurred between 7 a.m and 8 a.m on days when the animals did not participate in the Open Field test and between 7 a.m and 10:30 a.m on days when they were evaluated in the Open Field. After the last Open Field test on day 71 of treatment, the animals were euthanized, and the striatum was extracted.
2.3. Pharmacological treatment
The treatment consisted of a saline vehicle at 0.9% (0.1 mL/100 g) for the control group and pramipexole (0.1 mg/kg, dissolved in the saline solution at 0.9%) for the drug group and was always administered at the same time of day for 71 days [8]. On days when rats were evaluated in the Open Field, the dose was injected into the peritoneal cavity 10 min before the beginning of the test.
2.4. Open Field
The exposure to the Open Field [9] was performed between 9 a.m. and 11 a.m., and each animal was individually placed in the center of the apparatus and observed for 10 min. However, only the final 5 min was evaluated. The Open Field consisted of a circular wooden ring measuring 81 cm in diameter and enclosed by 41 cm-high white walls. The ceiling was open, and the ground was divided into 19 quadrants. In line with the circadian pattern of human RLS symptoms, which appear or worsen during the night, all tests were performed between 9 a.m. and 11 a.m. because rats are nocturnal animals [10]. During the experiment, the rats were evaluated for peripheral ambulation (number of quadrants that the animal stepped on with four paws near the walls of the apparatus), central ambulation (number of quadrants that the animal stepped on with four paws that were not close to the walls of the apparatus), total ambulation (the total number of quadrants that the animal stepped on with four paws) rearing (the number of times that the animal supported itself with both hind legs), total duration of grooming (the total amount of time that the animal put its mouth or paws on its body or head) and immobility time (the total amount of time that the animal remained perfectly still, moving only the vibrissae) [11].
2.5. Western Blot
After decapitation, the striatum (caudate and putamen) was rapidly removed, and the fragments were frozen. During the dissection, the equipment was kept at a low temperature, and the biological material was stored at −80 °C until used. D2 dopaminergic receptor and the dopamine transporter (DAT) expressions were evaluated by Western Blot analysis. The tissue was homogenized in lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 100 mM Tris–HCl pH 8.0, 150 mM NaCl, 10 mM EDTA, 0.1% SDS, 10% glycerol, and Protease Inhibitor Cocktail (Sigma), incubated on ice for 10 min, centrifuged at 7000g for 5 min at 4 °C, and the supernatant was collected. Protein concentration was assayed using the bicinchoninic acid method (BCA, Pierce). Protein from each sample (20 µg) was subjected to SDS-PAGE and then transferred to a PVDF membrane. The membranes were incubated with blocking solution for 1 h (5% skimmed milk diluted in TBS-T (50 mM Tris pH 7.4, 150 mM NaCl, and 0.1% Tween-20), incubated with a primary antibody diluted in TBS-T (1:500) for 1 h, and washed 3 times with TBS-T for 5 min. After washing, membranes were incubated with the diluted anti-rabbit secondary antibody in TBS-T for 1 h (1:10,000) and washed five times with TBS-T for 5 min. Finally, the bands of interest were revealed with a chemiluminescent substrate (Pierce) and a digital imaging system (MF-ChemiBIS Imaging System). After image acquisition, quantification of proteins of interest was performed using the Totallab 100 (Nonlinear dynamics) software.
2.6. Statistical analysis
The experiments were evaluated with two-way analysis of variance (ANOVA) with repeated measures followed by post-hoc Tukey׳s post-hoc test. The non-parametric Mann–Whitney test was used for the D2 receptor analyses. The results are expressed as the mean±standard error of the mean (SEM). The level of significance was set at p<0.05.
3. Results
3.1. Immobility time
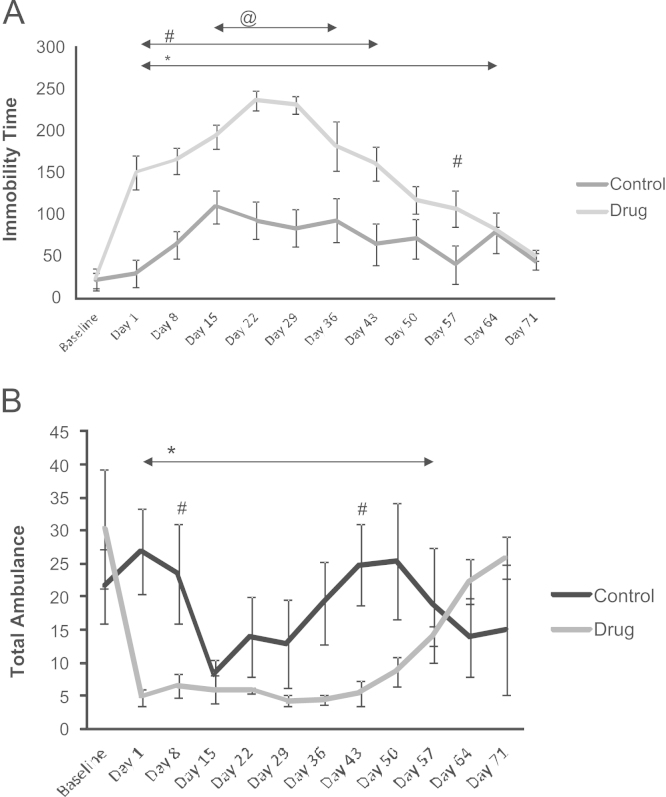
ANOVA for the repeated measures revealed significant effects for the factors groups (F(1.15)=24.027, p<0.001) and time (F(11.165)= 14.326, p<0.001) and the interaction of groups×time (F(11.165)= 4.841, p<0.001). Fig. 1A shows that the drug group exhibited a significant increase in the immobility time compared to baseline from day 1 to 64 of the treatment. It also shows a significant increase in the immobility time for the drug group compared to baseline from days 15 to 36 of the treatment. Moreover, the drug group exhibited a significant increase relative to the control group between days 1 and 43 and on day 57 of the treatment. The control group exhibited a significant increase in immobility time relative to baseline from days 15 to 36 of the treatment.
Fig. 1.
(A) Assessment in the Open Field of immobility time in the control (saline) and drug (pramipexole) groups. * indicates a significant increase in the immobility time of the drug group compared to the baseline level from days 1 to 64 (ANOVA. p<0.05); @ indicates a significant increase in the immobility time of the control group relative to the baseline level from days 15 to 36 of the treatment (ANOVA, p<0.05); # indicates the same day differences between the groups between days 1 and 43 and day 57 of treatment (ANOVA, p<0.05). (B) Assessment in the Open Field of total ambulation (number of quadrants) by the control (saline) and drug (pramipexole) groups. * indicates a significant reduction in total ambulation in the drug group compared to baseline from days 1 to 57 (ANOVA, p<0.05); # indicates same day differences between the groups on days 8 and 43 of treatment (ANOVA, p<0.05). H s: hundred seconds.
3.2. Total ambulation
ANOVA for repeated measures revealed significant effects for the factor time (F(11.165)=2.7735, p<0.001), and the interaction groups×time (F(11.165)=3.9991, p<0.001). Fig. 1B shows that the drug group exhibited a significant reduction in total ambulation relative to baseline from days 1 to 57 of the treatment. In addition, the control group exhibited a significant increase in total ambulation compared to the drug group on days 8 and 43 of treatment.
3.3. Rearing
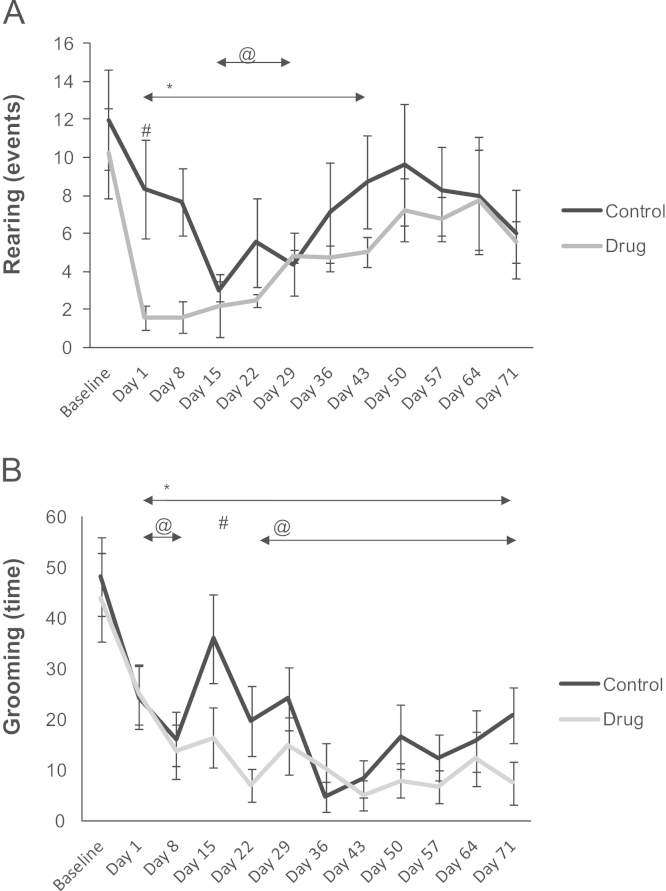
ANOVA for repeated measures revealed a significant effect for the factor time (F(11.165)= 3.8419, p<0.001). Fig. 2A shows that the drug group demonstrated a significant decrease in rearing relative to baseline from days 1 to 43 of the treatment. This figure also shows that the control group exhibited a significant decrease in rearing in relative to baseline from days 15 to 29 of the saline solution treatment. The control group exhibited a significant increase compared to the drug group on day 1 of the treatment.
Fig. 2.
(A) Assessment of rearing of Wistar rats in the control (saline) and drug (pramipexole) groups in the Open Field. * indicates a significant decrease in rearing in the drug group compared to the baseline level from days 1 to 43 of treatment (ANOVA, p<0.05); @ indicates a significant decrease in rearing in the control group relative to baseline from days 15 to 29 of the treatment (ANOVA, p<0.05); # indicates a same day difference between the groups on day 1 of treatment (ANOVA, p<0.05). B assessment of grooming (time, seconds) in the Open Field in the control (saline) and drug (pramipexole) groups. * indicates a significant decrease in grooming in the drug group compared to baseline from days 1 to 71 of treatment (ANOVA, p<0.05); @ indicates a significant decrease in grooming in the control group relative to baseline from days 1 to 8 and days 22 to 71 of treatment (ANOVA, p<0.05); # indicates a same day difference between the groups on day 15 of treatment (ANOVA, p<0.05).
3.4. Grooming (time)
ANOVA for repeated measures revealed significant effects for the factors groups (F(1.15)= 6.441, p<0.02273) and time (F(11.165)= 6.767, p<0.001). Fig. 2B shows that the drug group demonstrated a significant decrease in grooming relative to baseline from days 1 to 71 of the treatment. It is noteworthy that the control group showed a significant decrease in grooming relative to baseline from days 1–8 to days 22–71 of the treatment. There were also significant differences between the groups on day 15 of the treatment.
3.5. D2 receptor expression
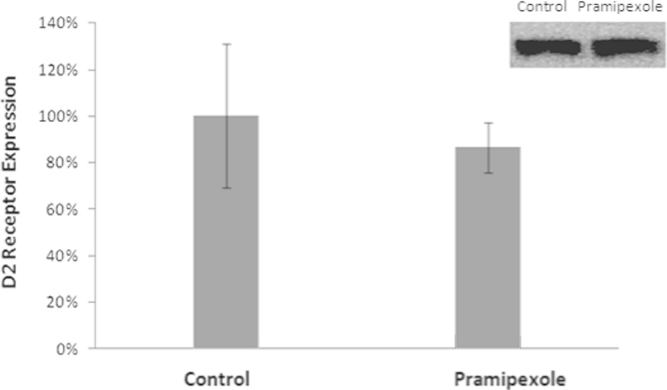
The results showed no significant differences in the expression of the dopamine D2 receptor between the control and drug groups after 71 days of treatment (Fig. 3).
Fig. 3.
Analysis of the relative expression of the D2 dopamine receptor in the control and drug (Pramipexole) groups (Mann–Whitney, p>0.05).
4. Discussion
This study aimed to assess the long-term effects of dopaminergic treatment (PPX) on locomotor activity and their relationship with the expression of the D2 receptor.
Pramipexole (PPX) is a non-ergot agonist of D2/D3 that is used for the treatment of RLS and Parkinson׳s disease [12]. The increased effectiveness of this medication has been demonstrated by numerous multicenter studies [13]. However, many patients have experienced residual symptoms during treatment, and from 12% to 25% of patients discontinue use due to either side effects or the loss of efficacy [1]. Ferini-Strambi [14] estimated that 8.3% of 60 patients treated for 6 months with dopamine agonists showed augmentation. In another study with a mean duration of 27.2 months, Silber et al. [15] found that 33% of the 49 patients developed augmentation after 13.8 months of treatment. García-Borreguero et al. [16] suggested that 20% of patients develop augmentation after 1 year of treatment, and the rate increases to 30% after 2 years.
Currently, the precise nature of augmentation is not fully understood. What is known is that it is caused by a disturbance of the dopaminergic system [5]. It is believed that the increased dopaminergic state is caused by excessive stimulation of D1 compared with D2 receptors, which leads to decreased sensitivity and a down-regulation of the D1 receptor. It is also believed that this hyper-dopaminergic state leads to a supersensitivity of the dopamine receptor, similar to a mechanism proposed for tardive dyskinesia. Another theoretical possibility based on excessive orexinergic stimulation states that this stimulation would lead to a hyper-motor syndrome via the large projections of orexinergic neurons to the monoaminergic system and the anterior horn cells of the spinal cord [4].
The results of this study showed that animals treated with PPX for 71 days initially exhibited reduced locomotor activity and increased immobilization, and after 64 days, these behavioral characteristics started to return to baseline levels.
Using PET, Cervenka et al. [17] demonstrated increased availability of D2 receptors in patients with RLS. This interpretation is consistent with the dopaminergic neurotransmission hypothesis in RLS, wherein the increased concentration of receptors may be due to the positive regulation of the receptor in response to low concentrations of dopamine. Interestingly, sustained treatment with PPX (which over stimulates somatodendritic D2/D3 receptors) can lead to a decrease in D2/D3 receptor responsiveness and a subsequent restoration of the mean firing rate of DA neurons [7]. The present study showed no significant difference in D2-like receptor expression due to long-term PPX administration (71 days). However, it is known that continuous stimulation during treatment down-regulates the number of receptors, and, by analyzing Fig. 3, there seems to be a tendency for a reduction of D2 receptor expression. This finding is consistent with results previously reported by Chernoloz et al. [7] that demonstrated a decreased sensitivity and density of D2-like receptors following chronic administration of the D2-like agonist PPX
Studies have shown that the development of regular locomotion, requires a synergistic interaction between the dopaminergics D1 and D2 [18] or D1 and D3 receptors [19,20]. However, activation of the postsynaptic D2 receptors can increase locomotion. In the hyper-dopaminergic phase of RLS treatment, hyper-excitability of the dopaminergic system leads to a reduction of the flexor reflexes and an initial increase of the delayed flexor reflexes [5]. This result was also confirmed in the present study because during the initial phase of the PPX treatment, the rats showed a significant reduction in locomotion and a substantial increase in immobilization.
The present study has some limitations. Despite relatively long-term treatment, our animals did not demonstrate evidence of augmentation (by the Open Field test). This may be due to possible tolerance acquired during the long treatment period. Thus, an increase in the drug dose might have demonstrated the augmentation that usually occurs in humans.
In summary, the present results demonstrate the effects of long-term dopaminergic treatment on locomotor behavior in rats. Rats that received PPX treatment for 71 days showed an immediate reduction in locomotion and an increase in immobilization, and after 64 days, these values returned to baseline levels without evidence of augmentation. In addition, under these conditions it was not possible to demonstrate the relationship between locomotor activity and the expression of D2 receptors.
Thus, for future studies, it is important consider experiments using spontaneously hypertensive rats, an animal model of RLS previously reported by Esteves et al., [21], to investigate the underlying pathophysiology of augmentation.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by grants from Associação Fundo de Incentivo à Pesquisa (AFIP), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2011/12540-4 to WAOA and CEPID No. 98/14303-3 to ST). The authors thank the Centro de Estudos em Psicobiologia e Exercício (CEPE) and Centro de Estudos Multidisciplinar em Sonolência e Acidentes (CEMSA). ST and MTM are recipients of the CNPq fellowship.
References
- 1.Earley C.J., Silber M.H. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med. 2010;11(9):807–815. doi: 10.1016/j.sleep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Allen R.P., Earley C.J. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205–213. doi: 10.1093/sleep/19.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Winkelman J.W., Johnston L. Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS) Sleep Med. 2004;5:9–14. doi: 10.1016/j.sleep.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Chokroverty S. Long-term management issues in restless legs syndrome. Mov. Disord. 2011;26:1378–1385. doi: 10.1002/mds.23652. [DOI] [PubMed] [Google Scholar]
- 5.Paulus W., Schomburg E.D. Dopamine and the spinal cord in Restless Legs Syndrome: does spinal cord physiology reveal a basis of augmentation? Sleep Med. Rev. 2006;10:185–196. doi: 10.1016/j.smrv.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Ondo W., Romanyshyn J., Vuong K.D., Lai D. Long-term treatment of restless legs syndrome with dopamine agonists. Arch. Neurol. 2004;61:1393–1397. doi: 10.1001/archneur.61.9.1393. [DOI] [PubMed] [Google Scholar]
- 7.Chernoloz O., El Mansari M., Blier P. Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology. 2009;34(3):651–661. doi: 10.1038/npp.2008.114. [DOI] [PubMed] [Google Scholar]
- 8.Luo F., Li C., Ondo W.G., Xu P., Xie W., Le W. The long-term effects of the dopamine agonist pramipexole in a proposed restless legs syndrome animal model. Sleep Med. 2011;12:41–46. doi: 10.1016/j.sleep.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Broadhurst P.L. Experiments in psychogenetics: applications of biometrical genetics to the inheritance of behaviour. In: Eysenck H.J., editor. vol. 1. Routledgc & Kegan Paul; London: 1960. (Experiments in Personality). [Google Scholar]
- 10.Qu S., Le W., Zhang X., Xie W., Zhang A., Ondo W.G. Locomotion is increased in A11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J. Neuropathol. Experimental Neurol. 2007;66:383–388. doi: 10.1097/nen.0b013e3180517b5f. [DOI] [PubMed] [Google Scholar]
- 11.Fukushiro D.F., Calzavara M.B., Trombin T.F, Lopez G.B., Abílio V.C., Andersen M.L., Tufik S., Frussa-Filho R. Effects of environmental enrichment and paradoxical sleep deprivation on open-field behavior of amphetamine-treated mice. Physiol. Behav. 2007;92:773–779. doi: 10.1016/j.physbeh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Becker P.M., Ondo W., Sharon D. Encouraging initial response of restless legs syndrome to pramipexole. Neurology. 1998;51:1221–1223. doi: 10.1212/wnl.51.4.1221. [DOI] [PubMed] [Google Scholar]
- 13.Scholz H, Trenkwalder C, Kohnen R, Kriston L, Riemann D, Hornyak M. Dopamine agonists for the treatment of restless legs syndrome. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No.: CD006009. DOI: 10.1002/14651858.CD006009.pub2. [DOI] [PMC free article] [PubMed]
- 14.Ferini-Strambi L. Restless legs syndrome augmentation an pramiprexole treatment. Sleep Med. 2002;3:S23–S25. doi: 10.1016/s1389-9457(02)00144-2. [DOI] [PubMed] [Google Scholar]
- 15.Silber M., Girish M., Izurieta R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003;26:819–821. doi: 10.1093/sleep/26.7.819. [DOI] [PubMed] [Google Scholar]
- 16.García-Borreguero D., Allen P., Benes H., Earley C., Happe S., Högl B., Kohnen R., Paulus W., Rye D., Winkelmann J. Augmentation as a treatment complication of restless legs syndrome: concept and management. Mov. Disord. 2007;22:476–484. doi: 10.1002/mds.21610. [DOI] [PubMed] [Google Scholar]
- 17.Cervenka S., Palhagen S.E., Comley R.A., Panagiotidis G., Cselenyi Z., Matthews J.C., Lai. R.Y., Halldin C., Farde L. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129:2017–2028. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 18.Fleetwood-Walker S.M., Hope P.J., Mitchell R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurons. J. Physiol. 1988;399:335–348. doi: 10.1113/jphysiol.1988.sp017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartesveldt C.V. Temporal and environmental effects on quinpirole-induced biphasic locomotion in rats. Pharmacol. Biochem. Behav. 1997;58:955–960. doi: 10.1016/s0091-3057(97)00332-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang X., Oosting R.S., Jones S.R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc. Natl. Acad. Sci. USA. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteves A.M., Lopes C., Frussa-Filho R., Frank M.K., Cavagnolli D., Arida R.M., Tufik S., de Mello M.T. Spontaneously hypertensive rats: possible animal model of sleep-related movement disorders. J. Mot. Behav. 2013;45(6):487–493. doi: 10.1080/00222895.2013.833079. [DOI] [PubMed] [Google Scholar]