Abstract
Introduction
Measles, mumps, rubella, and varicella combination vaccines (MMRV) facilitate varicella vaccination uptake compared with separate administration of measles, mumps, and rubella vaccine (MMR) with varicella vaccine (V). However, the risk of developing febrile convulsions (FC) is higher in children vaccinated with MMRV.
Objectives
The aim was to demonstrate how to put the increased FC risk associated with MMRV into perspective by comparing it with the lower V-coverage risk associated with MMR + V.
Methods
FC and varicella burdens were measured by total numbers or duration of hospitalisations. A model, based on several assumptions and integrating parameters from heterogeneous data sources relevant to Germany, was developed to evaluate hospitalisation ratios (HRs; ratios between yearly numbers of varicella-related hospitalisation days prevented by MMRV and yearly numbers of FC-related hospitalisation days attributed to MMRV, both compared with MMR + V). A sensitivity analysis estimated HR under different scenarios beyond the German experience.
Results
For parameter values compatible with the German experience, where MMRV (Priorix-Tetra™, GSK, Belgium) was introduced in 2006, the model predicted that transitioning from MMR + V to MMRV would induce 225 vaccine-related FC hospitalisation days whilst preventing 1976 varicella-related hospitalisation days per year. The HR estimated by Monte Carlo simulations was 8.5 (95 % confidence interval: 1.99–25.22). A sensitivity analysis on two key parameters suggested that transitioning from MMR + V to MMRV would be favourable in situations where MMRV use would significantly impact varicella vaccination uptake.
Conclusions
MMRV use instead of MMR + V can substantially reduce the number of hospitalisation days, despite increased FC risk when MMRV is used as a first dose of measles-containing vaccine.
Key Points
| Our modelling suggests that the use of measles, mumps, rubella, and varicella combination vaccines (MMRV) instead of measles, mumps, and rubella vaccine with varicella vaccine (MMR + V) can substantially reduce the number of hospitalisation days via higher vaccination coverage against varicella, despite the observed increased risk of febrile convulsions when MMRV is used as a first dose of measles-containing vaccine. |
| The net result of these two opposing effects is one of the trade-offs between the two vaccination schemes that needs to be considered when making decisions on their use in immunisation programmes. |
| This proof-of-concept analysis has demonstrated the feasibility and usefulness of quantitative modelling approaches, based on the combination of heterogeneous data sources, to provide objective, rational, and transparent information. |
Introduction
Combination vaccines are used to simplify the recommended immunisation schedules, decrease the number of healthcare visits and injections in children, and improve vaccination coverage and compliance [1, 2]. Therefore, measles, mumps, rubella, and varicella combination vaccines (MMRV) have been developed to replace the separate administration of the trivalent measles, mumps, and rubella vaccine (MMR) with the monovalent varicella vaccine (V). As seen with other combination vaccines, MMRV has been a key driver in improving the vaccination coverage against varicella in various countries, such as Germany and the USA [3–8].
German data suggest that V uptake was suboptimal when separate administration of MMR + V was recommended. Indeed, some parents are willing to vaccinate their child against MMR, but do not accept separate V and MMR vaccination. The main factors associated with the low acceptance rate of V vaccination by parents are the recommendations of paediatricians, who often have doubts about the benefits of varicella vaccination as they rarely observe severe complications in their practices; a negative attitude towards vaccination in general; parents doubts about vaccine safety and effectiveness; and the perception that varicella is a mild disease [5, 7, 8]. Data from Germany, where MMRV was introduced in 2006, also showed that, even when MMRV was widely used, varicella vaccination coverage and timeliness of vaccination could still be improved [9]. Data from a national sentinel network led by the Robert Koch Institute suggested that varicella vaccination coverage in Germany was 78 % in 2008 [10]; however, this could be an overestimation of the real coverage since the network may not be representative of the entire country [6].
In children, the risk of febrile convulsions (FC) is increased during the first 2 weeks following MMR vaccination [11, 12]. In addition, more recent studies have shown that this risk was about two times higher in children vaccinated with the first MMRV vaccine licensed worldwide (ProQuad®, Merck & Co., Inc., Whitehouse Station, New Jersey, USA) compared with the separate administration of MMR + V [13, 14]. Later, a study conducted by the Bremen Institute for Epidemiology and Prevention Research showed that the risk of developing FC within 5–12 days post-vaccination was also higher in children younger than 5 years who received the other licensed MMRV vaccine (Priorix-Tetra™, GSK, Belgium) compared with separate injections of MMR + V or MMR alone [15]. A further study provided similar risk estimates from Canada [16]. These observations suggest that both MMRV vaccines, when used as a first dose of measles-containing vaccine, are associated with an increased risk of FC [17].
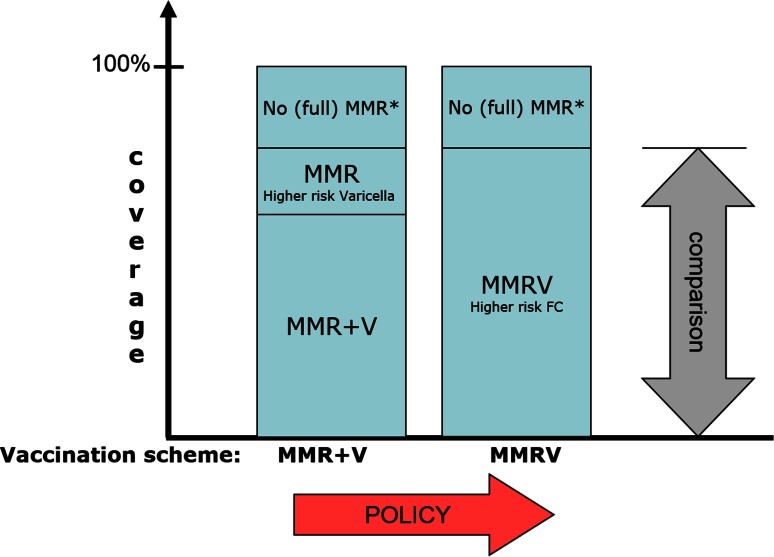
While combined MMRV vaccines can potentially increase the number of FC cases, they can decrease the number of varicella cases through increased coverage for the V component. Here, we present an attempt to assess the net result of these two opposing effects (Fig. 1). This comparison would ideally be made by counting the hospitalisations for FC and varicella, and their duration, in a number of large populations randomly allocated to MMRV and MMR + V schemes, over several years, and with everything else being equal. As this is hardly feasible, we selected a modelling approach and the recent experience in Germany as a starting point. In Germany, a general varicella immunisation for infants from the age of 11 months was introduced in 2004, followed by the subsequent recommendation of a second vaccine dose at 15–23 months of age in 2009. Priorix-Tetra™ was licensed in July 2006, and both vaccination schemes (MMR + V and MMRV) have been widely used; this has favoured comparison between the two vaccination regimens in a previous study conducted by the Bremen Institute for Epidemiology and Prevention Research [15]. In our analysis, we first used German data to put the increased risk of FC associated with MMRV (Priorix-Tetra™) into perspective by comparing it with the higher risk of varicella infection associated with the MMR + V regimen. Then, we expanded this analysis to other scenarios beyond the German experience.
Fig. 1.
Schematic representation of the two competing vaccination schemes and their associated risks and benefits as selected for the current analysis. *The (small) fraction of the total population who is not properly vaccinated against MMR. FC febrile convulsions, MMR measles, mumps, and rubella vaccine, MMRV measles, mumps, rubella, and varicella vaccine, V varicella vaccine
Methods
In this quantitative analysis, the increased risk of FC associated with MMRV (Priorix-Tetra™) was contrasted with the higher risk of varicella infections associated with the separate MMR + V vaccination approach (Fig. 1). We used a model based on several assumptions to integrate parameters from heterogeneous sources of data from Germany (in the period 2006–2008). Statistical uncertainty was evaluated through a probabilistic uncertainty analysis by Monte Carlo simulations. In addition, to address the potential variability between countries or over time, structural uncertainty was assessed through a sensitivity analysis.
The assumptions, as well as the point estimates for the input parameters, are described in Table 1, and were selected to mimic the situation in Germany in the period 2006–2008. The risk estimates were derived from the post-marketing study conducted by the Bremen Institute for Epidemiology and Prevention Research among children who received a first vaccination with MMRV (Priorix-Tetra™), MMR, or MMR + V between January 2006 and December 2008 [15]. Whereas the German vaccination programme changed to a two-dose schedule for MMRV or MMR + V in 2009, only the first doses were taken into account in this population-based analysis.
Table 1.
Definitions and point estimates of the input parameters, and uncertainty of these parameters observed in the Monte Carlo simulations
| Parameter | Point estimate | Sources | Distribution | Simulation, median (95 % CI) | |
|---|---|---|---|---|---|
| P1 | Incidence of FC in days 5–12 after MMRV (number of FC per child) | 51/82,436 | [15] | Poisson | 61.9 (46.1–78.9)a |
| P2 | Incidence of FC in days 5–12 after MMR (number of FC per child) | 21/82,469 | [15] | Poisson | 25.5 (15.8–36.4)a |
| P3 | Relative probability of hospitalisation for FC as compared with the German data from 2006–2008 | 1.0 | [15] | Constant | 1 (–) |
| P4 | Number of hospitalisations for varicella per year (before the introduction of routine vaccination against varicella, across all age groups) | 1996 | [24] | Normal | 1998 (1365–2633) |
| P5 | Median LOS for FC (number of days) | 1 | [26] | Constant | 1 (–) |
| P6 | Median LOS for varicella (number of days) | 5 | [24] | Poisson | 5 (1–10) |
| P7 | MMR coverage for the first dose | 0.9 | [8] | Constant | 0.9 (–) |
| P8 | Probability of V vaccination along with MMR vaccination | 0.78 | [10] | Constant | 0.78 (–) |
| P9 | German birth cohort size (year 2005) | 685,795 | National statistics | Constant | 685,795 (–) |
CI confidence interval, FC febrile convulsions, LOS length of stay in hospital, MMR measles, mumps, and rubella vaccine, MMRV measles, mumps, rubella, and varicella vaccine, P parameter, V varicella vaccine
aCumulative incidence per 100,000 children
The primary health outcome in this analysis was the duration of hospitalisation (median length of stay), which was chosen as a measure of the burden of both FC and varicella infection. Yearly attributable numbers of hospitalisation days were estimated for each vaccination regimen. The secondary health outcome in this analysis was the number of hospitalisations.
Modelling Assumptions
The model was based on the following assumptions:
Vaccination with V occurred only in co-administration with MMR.
MMR vaccination coverage and effectiveness were considered as identical between the MMR + V and the MMRV regimens.
Vaccination coverage of V varied between the two vaccination regimens.
Vaccine effectiveness of V was considered identical under both vaccination regimens [18–20].
One dose of V conferred identical and total protection against varicella-related hospitalisation under both vaccination regimens [21].
The two vaccination regimens were compared as if they had been implemented for a sufficient amount of time as to reach a steady state in the dynamics of the disease, and all effects were assumed to be linear [22].
The incidence of adverse events leading to hospitalisation, other than FC in the period of 5–12 days after the first dose, were considered identical under both vaccination regimens [18, 23].
There were no health consequences later in life from hospitalisation for FC or varicella [24, 25].
The number of hospitalisations for varicella after introduction of routine varicella vaccination was linearly proportional to the proportion of unvaccinated children.
Calculation of the Hospitalisation Ratio
To quantify the benefit, we calculated the number of hospitalisation days and the number of hospitalisations for varicella that could be averted when using MMRV instead of MMR + V. The hospitalisation rate for varicella in unvaccinated children was based on the yearly number of varicella-related hospitalisations observed in Germany before the introduction of routine varicella vaccination. The yearly number of varicella-related hospitalisation days and hospitalisations attributable to MMR + V compared with MMRV were estimated as follows (Table 1): (P6) × (P4) × (P7) × [1 − (P8)] and (P4) × (P7) × [1 − (P8)], respectively.
To quantify the risk, we calculated the yearly number of hospitalisation days and the number of hospitalisations for FC that were attributable to MMRV compared with MMR or MMR + V. The yearly population at risk was the fraction of the birth cohort vaccinated with MMRV. The yearly number of hospitalisation days and hospitalisations for FC attributable to MMRV compared with MMR + V were estimated from the differences between the incidence of FC under both vaccination regimens as follows (Table 1): (P5) × (P9) × (P7) × [(P1) − (P2)] × (P3) and (P9) × (P7) × [(P1) − (P2)] × (P3), respectively.
The hospitalisation ratio (HR) was the ratio between the excess number of hospitalisation days (primary outcome) or hospitalisations (secondary outcome) attributable to varicella when vaccinating with MMR + V compared with MMRV, and the excess number of hospitalisation days (primary outcome) or hospitalisations (secondary outcome) attributable to vaccine-related FC when vaccinating with MMRV compared with MMR + V.
Probabilistic Uncertainty Analysis
The statistical variability of each non-constant input parameter of the model was used to evaluate the statistical uncertainty of the HR through a probabilistic analysis based on Monte Carlo simulations. The most relevant statistical distribution was selected for each input parameter, and 100,000 sets with randomly generated values for all input parameters were produced. The point estimate from the Monte Carlo simulation was defined as the median of the 100,000 values, and the 95 % confidence interval (CI) was derived from the observed percentiles.
Rather surprisingly, data from the Bremen Institute for Epidemiology and Prevention Research indicated a median duration of hospitalisation for FC of 2–3 days during the first month following MMR or MMRV vaccination in 2006–2008, with several cases having a relatively long duration of up to 17 days. However, since the most recent German guidelines include no indication on whether children with FC should be hospitalised or not, the duration of hospitalisation for FC was based on the most recent international recommendations [26] and set to a value of 1 day in this analysis (Table 1). A study conducted in 1985 in the UK already suggested that children who had been observed for 24 h after FC could be discharged from hospital if the diagnosis of the cause of fever had been established and the children were medically fit, even if they were still febrile [27]. With current vaccination schedules, the risk of meningitis has become extremely low, and it is not recommended anymore to carry out unnecessary investigations in most children presenting with FC [26].
Current international recommendations for the management of FC advocate minimal intervention [26–29]. In Germany, the Bremen Institute for Epidemiology and Prevention Research reported that hospitalisation rates for FC in 2006–2008 were around 60 % in 9- to 17-month-old children; this estimation was based on different case definitions for hospitalised and non-hospitalised children, and did not take into account the timing of MMR or MMRV vaccination. In a study conducted in the USA, hospitalisation rates for FC were 6 and 17 % during 7–10 days following MMRV and MMR + V vaccination, respectively [14]. In another study, hospitalisation rates dropped significantly after implementation of updated guidelines for the management of children with FC (from 57.3 to 20.5 % and from 16.9 to 3.2 % in two large hospitals in France and Italy, respectively) [28].
Sensitivity Analysis
We identified the two parameters that are most likely to vary largely between countries or over time and have a large effect on the HR: rates of hospitalisation for FC and vaccination coverage for V under MMR + V. A sensitivity analysis was conducted to explore the structural uncertainty of HR in situations where the values of these two parameters were changed over their potential range. Monte Carlo simulations were used to derive the different values of both the relative probability of hospitalisation for FC as compared with the German data from 2006–2008 (parameter P3) and the vaccination coverage for V under MMR + V (parameter P8), for which the point estimates for the HR were 1. Statistical uncertainty was estimated as explained above.
In the study of the Bremen Institute for Epidemiology and Prevention Research, the probability of hospitalisation for FC was 100 % by definition since only hospitalised cases were included in the analysis [15]. However, in the German national context, the probability of hospitalisation for FC is not 100 % and is considered to be close to 80 %. To allow for generalisation, the model was slightly modified in order to use the national probability of hospitalisation for FC instead of the one related to the specific study of the Bremen Institute for Epidemiology and Prevention Research.
In order to differentiate the parameter space where the HR could be considered to be significantly higher than 1, we also derived the parameter values for which the lower limit of the 95 % CI around the HR was 1.
Results
Modelling Based on the German Experience
The estimates for the different input parameters used in the model were selected from heterogeneous sources of data as described in Table 1 [8, 10, 15, 24]. When using these point estimates for all parameters, the model predicted 1976 days of hospitalisation (395.4 hospitalisations) for varicella averted when the MMRV vaccination regimen was used, compared with 225 days of hospitalisation (225.0 hospitalisations) for FC attributable to MMRV. The point estimate of the HR was close to 11 in the primary analysis, which was based on the number of hospitalisation days.
When the random variability of each non-constant input parameter of the model was estimated by a Monte Carlo probabilistic uncertainty analysis, the median HR was 8.48 (95 % CI: 1.99–25.22) in terms of hospitalisation days and 1.76 (0.98–4.02) in terms of number of hospitalisations (Table 2). The difference with the point estimate presented above may be explained by the non-symmetrical distribution of the HR. In the primary analysis, the lower limit of the 95 % CI around the HR was above 1, indicating that the number of hospitalisation days attributable to non-prevented varicella when using MMR + V as compared with MMRV was significantly higher than the number of hospitalisation days attributable to FC after MMRV as compared with MMR + V at a 95 % CI (Table 2).
Table 2.
Benefit and risk estimates in terms of number of hospitalisation days in Germany
| Name | Mean | Median | 95 % CI |
|---|---|---|---|
| Yearly number of hospitalisation days for FC attributable to MMRV (risk) | 225.0 | 224.7 | 104.9–351.9 |
| Yearly number of hospitalisation days for varicella prevented under MMRV (benefit) | 1989 | 1879 | 433.4–4125 |
| Hospitalisation ratio | 8.5 | 1.995–25.22 |
CI confidence interval, FC febrile convulsions, MMRV measles, mumps, rubella, and varicella vaccine
Sensitivity Analysis
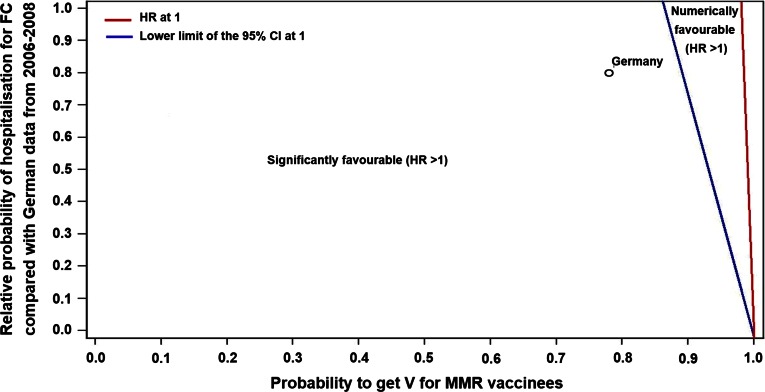
Expected outcomes in other countries or under different scenarios were derived from a sensitivity analysis, which was based on two key parameters: the vaccination coverage of V when co-administered with MMR and the probability of being hospitalised for FC. The point estimate of the HR in terms of hospitalisation days was higher than 1 for scenarios where the probability of being hospitalised for FC was less than 40 times the probability of not receiving V along with MMR (Fig. 2).
Fig. 2.
Estimates of the HR in terms of number of hospitalisation days under different scenarios. CI confidence interval, FC febrile convulsions, HR hospitalisation ratio, MMR measles, mumps, and rubella vaccine, V varicella vaccine
In countries where the mean duration of hospitalisation for varicella was different than in Germany (e.g. 3 days instead of 5 days), the slope of the discriminating line, which is shown in Fig. 2, would be corrected by the same ratio (e.g. 40 × 3/5 = 24). When considering the line for the 95 % CI limit in Fig. 2, the main driver was the probability of not receiving V along with MMR.
Discussion
The results of our analysis using German data suggested that the predicted number of hospitalisation days induced by the increase risk of FC associated with MMRV is lower than that induced by higher risk of varicella infection associated with the MMR + V regimen. The expansion of this analysis to other scenarios beyond the German experience suggested that transitioning from MMR + V to MMRV would be favourable in situations where MMRV use would significantly impact varicella vaccination uptake.
For parameter values compatible with the recent experience in Germany, the model suggested that transitioning from MMR + V to MMRV would reduce the yearly number of hospitalisation days; although there may be 225 hospitalisation days attributable to vaccine-related FC, at the same time, 1976 hospitalisation days due to severe varicella could be averted by using MMRV instead of MMR + V. When Monte Carlo simulations were used to evaluate the statistical uncertainty, the median HR was estimated to be 8.5 (95 % CI 1.99–25.22). Since these estimations were derived from a model based on several assumptions and using heterogeneous sources of data, this quantitative analysis should be used along with other relevant considerations for any overall benefit/risk assessment of MMRV compared with MMR + V.
A sensitivity analysis was performed to estimate HR under different scenarios, which were based on existing or hypothetical situations, and could represent other countries or other times when vaccine recommendations or use were different. This modelling approach, with all its limitations, suggested that, in situations where MMRV use would significantly impact varicella vaccination uptake, the use of MMRV instead of MMR + V would be favourable. For any further use of these results, it is critical to consider the model assumptions and assess their relevance before making any decision on the use of MMRV in immunisation programmes.
The results are based on a modelling approach with several limitations, including:
The model did not consider situations where V was co-administered with the second MMR dose (either as MMR + V or MMRV) if it was not co-administered with the first MMR dose.
The assumption of total protection against hospitalisation could have overestimated the benefit measure of the MMRV compared with the MMR + V vaccination regimen.
The measures of the burden of both FC and varicella infection that were selected in this analysis (days of hospitalisation and number of hospitalisations) may not have completely captured the severity of the two medical conditions; hospitalisation for FC is generally more a measure of precaution, while hospitalisation related to varicella infection generally indicates a serious medical concern.
The model did not take into account the potential impact of the recommendation to administer the first doses of MMR and V separately in Germany since 2011 [3].
The model did not take into account the effect of herd immunity, which is relevant in the context of protection against varicella. This is a conservative approach since such an effect would increase the benefit of MMRV compared with MMR + V.
Conclusion
A modelling approach can be used to estimate the overall impact of different vaccine regimens on some specific outcomes. The model used in this analysis suggested that transitioning from MMR + V to MMRV may substantially reduce the number of hospitalisation days, despite the observed increased risk of FC when MMRV was used as a first dose of measles-containing vaccine. This proof-of-concept analysis has demonstrated the feasibility and usefulness of quantitative modelling approaches, based on the combination of heterogeneous data sources, to provide objective, rational, and transparent information. However, all assumptions and limitations should be kept in mind. In addition, our model only assessed one of the multiple trade-offs between the two vaccination regimens that need to be considered when making decisions on their use in immunisation programmes. In the future, more extensive research could be performed to address unanswered questions, such as analyses using quality-adjusted life-years as an outcome instead of the length and number of hospitalisations, and analyses taking into account indirect effects, such as herd immunity or increased hesitancy to accept MMRV due to knowledge of the increased risk of FC.
Acknowledgments
The authors acknowledge Christophe Dessart and Iris Depaz for their help, input, and constructive criticism. The authors also thank Claire Verbelen (XPE Pharma and Science, on behalf of GSK Vaccines) for writing support and incorporation of comments received from the authors, and Ashmita Ravishankar (employee of the GSK group of companies) for publication management.
Trademarks
Priorix-Tetra is a trademark of the GSK group of companies. ProQuad is a registered trademark of Merck & Co., Inc.
Compliance with Ethical Standards
Conflicts of interest
Vincent Bauchau and Lionel Van Holle are employed by the GSK group of companies and own restricted shares in the company. Carine Cohen was employed by the GSK group of companies at the time of this study and owns restricted shares in the company.
Source of funding
GlaxoSmithKline Biologicals SA sponsored this analysis and was involved in all stages of the analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
References
- 1.Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J. 2006;25:507–512. doi: 10.1097/01.inf.0000222413.47344.23. [DOI] [PubMed] [Google Scholar]
- 2.Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J. 2007;26:496–500. doi: 10.1097/INF.0b013e31805d7f17. [DOI] [PubMed] [Google Scholar]
- 3.Streng A, Liese JG. Decline of varicella vaccination in German surveillance regions after recommendation of separate first-dose vaccination for varicella and measles-mumps-rubella. Vaccine. 2014;32:897–900. doi: 10.1016/j.vaccine.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 4.Lopez AS, Cardemil C, Pabst LJ, Cullen KA, Leung J, Bialek SR, et al. Two-dose varicella vaccination coverage among children aged 7 years—six sentinel sites, United States, 2006–2012. MMWR Morb Mortal Wkly Rep. 2014;63:174–7. [PMC free article] [PubMed]
- 5.Reuss AM, Feig M, Kappelmayer L, Siedler A, Eckmanns T, Poggensee G. Varicella vaccination coverage of children under two years of age in Germany. BMC Public Health. 2010;10:502. doi: 10.1186/1471-2458-10-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siedler A, Hecht J, Rieck T, Tolksdorf K, Hengel H. Varicella vaccination in Germany. A provisional appraisal in the context of MMR vaccination. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:1313–1320. doi: 10.1007/s00103-013-1789-z. [DOI] [PubMed] [Google Scholar]
- 7.Siedler A, Rieck T, Reuss A, Walter D, Poggensee G, Poethko-Muller C, et al. Estimating vaccination coverage in the absence of immunisation registers—the German experience. Euro Surveill. 2012;17(17):pii 20152. [DOI] [PubMed]
- 8.Streng A, Seeger K, Grote V, Liese JG. Varicella vaccination coverage in Bavaria (Germany) after general vaccine recommendation in 2004. Vaccine. 2010;28:5738–5745. doi: 10.1016/j.vaccine.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Streng A, Grote V, Carr D, Hagemann C, Liese JG. Varicella routine vaccination and the effects on varicella epidemiology—results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006–2011. BMC Infect Dis. 2013;13:303. doi: 10.1186/1471-2334-13-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohle M, Siedler A, Bader HM, Ludwig M, Heininger U, Von Kries R. Assessment of varicella vaccine effectiveness in Germany: a time-series approach. Epidemiol Infect. 2011;139:1710–1719. doi: 10.1017/S0950268810002815. [DOI] [PubMed] [Google Scholar]
- 11.Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345:656–661. doi: 10.1056/NEJMoa003077. [DOI] [PubMed] [Google Scholar]
- 12.Vestergaard M, Christensen J. Register-based studies on febrile seizures in Denmark. Brain Dev. 2009;31:372–377. doi: 10.1016/j.braindev.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen SJ, Ackerson BK, Sy LS, Tran TN, Jones TL, Yao JF, et al. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009;27:4656–4661. doi: 10.1016/j.vaccine.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 14.Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126:e1–e8. doi: 10.1542/peds.2010-0665. [DOI] [PubMed] [Google Scholar]
- 15.Schink T, Holstiege J, Kowalzik F, Zepp F, Garbe E. Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR + V vaccination. Vaccine. 2014;32:645–650. doi: 10.1016/j.vaccine.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald SE, Dover DC, Simmonds KA, Svenson LW. Risk of febrile seizures after first dose of measles-mumps-rubella-varicella vaccine: a population-based cohort study. Can Med Assoc J. 2014;186:824–829. doi: 10.1503/cmaj.140078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma SJ, Xiong YQ, Jiang LN, Chen Q. Risk of febrile seizure after measles–mumps–rubella–varicella vaccine: a systematic review and meta-analysis. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Czajka H, Schuster V, Zepp F, Esposito S, Douha M, Willems P. A combined measles, mumps, rubella and varicella vaccine (Priorix-Tetra): immunogenicity and safety profile. Vaccine. 2009;27:6504–6511. doi: 10.1016/j.vaccine.2009.07.076. [DOI] [PubMed] [Google Scholar]
- 19.Knuf M, Faber J, Barth I, Habermehl P. A combination vaccine against measles, mumps, rubella and varicella. Drugs Today. 2008;44:279–292. doi: 10.1358/dot.2008.44.4.1210755. [DOI] [PubMed] [Google Scholar]
- 20.Knuf M, Habermehl P, Zepp F, Mannhardt W, Kuttnig M, Muttonen P, et al. Immunogenicity and safety of two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Pediatr Infect Dis J. 2006;25:12–18. doi: 10.1097/01.inf.0000195626.35239.58. [DOI] [PubMed] [Google Scholar]
- 21.Quian J, Ruttimann R, Romero C, Dall’Orso P, Cerisola A, Breuer T, et al. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997–2005. Arch Dis Child. 2008;93:845–850. doi: 10.1136/adc.2007.126243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Effelterre T, Hogea C, Cohen C. Impact of varicella vaccination coverage upon varicella-related hospitalizations in France and Germany: projections from a dynamic transmission model. Boston: Vaccine Congress; 2008. [Google Scholar]
- 23.Leung JH, Hirai HW, Tsoi KK. Immunogenicity and reactogenicity of tetravalent vaccine for measles, mumps, rubella and varicella (MMRV) in healthy children: a meta-analysis of randomized controlled trials. Expert Rev Vaccines. 2015 doi: 10.1586/14760584.2015.1057572. [DOI] [PubMed] [Google Scholar]
- 24.Liese JG, Grote V, Rosenfeld E, Fischer R, Belohradsky BH, Von Kries R, et al. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatr Infect Dis J. 2008;27:119–124. doi: 10.1097/INF.0b013e3181586665. [DOI] [PubMed] [Google Scholar]
- 25.Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, et al. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004;292:351–357. doi: 10.1001/jama.292.3.351. [DOI] [PubMed] [Google Scholar]
- 26.Oluwabusi T, Sood SK. Update on the management of simple febrile seizures: emphasis on minimal intervention. Curr Opin Pediatr. 2012;24:259–265. doi: 10.1097/MOP.0b013e3283506765. [DOI] [PubMed] [Google Scholar]
- 27.Green AL, MacFaul R. Duration of admission for febrile convulsions? Arch Dis Child. 1985;60:1182–1184. doi: 10.1136/adc.60.12.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callegaro S, Titomanlio L, Donega S, Tagliaferro T, Andreola B, Gibertini GG, et al. Implementation of a febrile seizure guideline in two pediatric emergency departments. Pediatr Neurol. 2009;40:78–83. doi: 10.1016/j.pediatrneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften: Guideline of the German society for Neuropaediatrics (2008). http://www.awmf.org/uploads/tx_szleitlinien/022-007_S2_Diagnostische_Prinzipien_bei_Epilepsien_im_Kindesalter_abgelaufen.pdf. Accessed 26 Jun 2014.