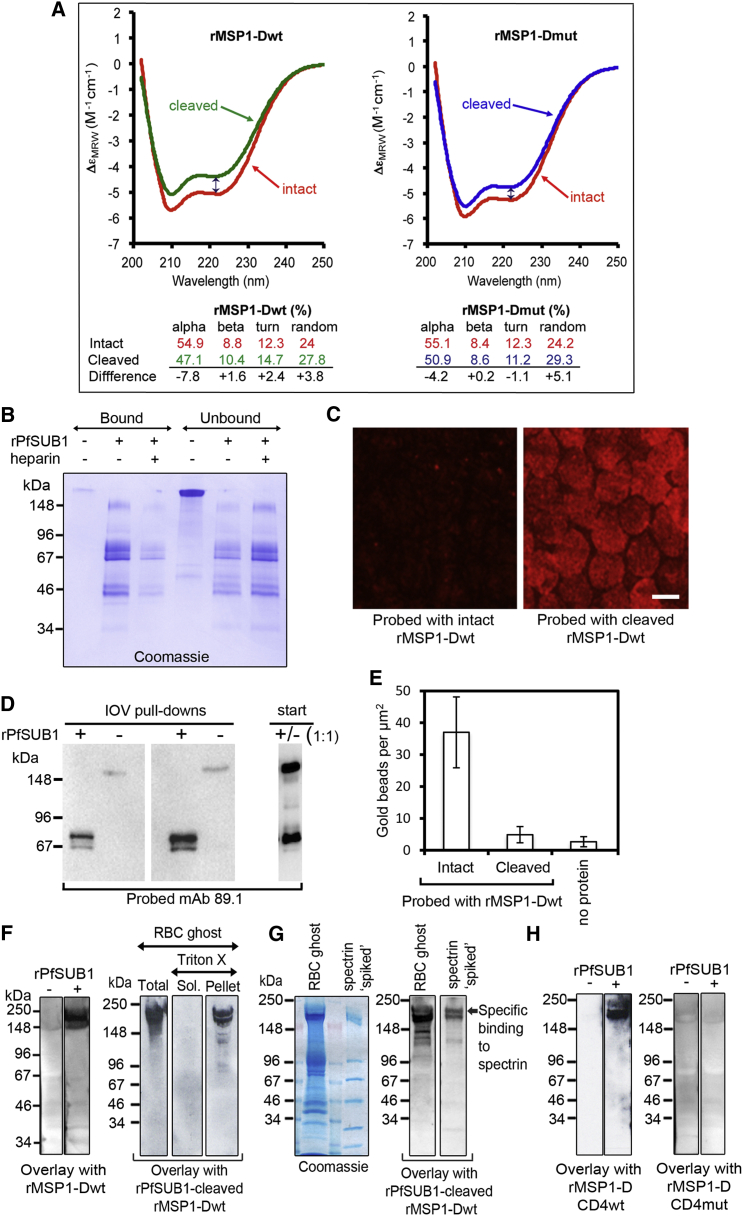
Figure 4.
Processing Alters MSP1 Secondary Structure and Activates a Heparin and Spectrin-Binding Activity
(A) Far-UV CD spectra of rMSP1-Dwt and rMSP1-Dmut as a function of molar absorptivity at 37°C. The vertical double arrow at 222 nm (negative minimum for the alpha helix spectrum) highlights the reduction in CD intensity following cleavage, which was 1.4-fold greater for rMSP1-Dwt than for rMSP1-Dmut. Below: secondary structure composition of the intact and processed proteins.
(B) Processing enhances heparin binding. Intact or cleaved rMSP1-Dwt was incubated with heparin agarose ± soluble heparin (1 mg ml−1), then binding assessed by SDS PAGE. Quantification of band intensity (Image Lab) showed that 72.3% ± 6.5% of cleaved rMSP1-Dwt bound heparin agarose but only 18.3% ± 12.1% of intact rMSP1-Dwt (p = 0.006, Student’s t test).
(C) Fixed, permeabilized erythrocytes probed with intact or rPfSUB1-cleaved rMSP1-Dwt. Binding was detected by IFA and imaged using equal exposure times. Mean pixel intensity (Adobe Photoshop Histogram tool) was 41.6 ± 4.7 (cleaved) and 12.6 ± 1.4 (uncleaved). Scale bar, 5 μm.
(D) Processing enhances binding to IOVs. Vesicles (∼80 μg protein) incubated with intact or rPfSUB1-cleaved rMSP1-Dwt (4 μg) were washed then two different loadings analyzed by western blot in parallel with a 1:1 mixture of the starting protein preparations.
(E) Processing enhances binding to the erythrocyte cytoskeleton. Mean density of bound gold beads following immunoEM of Triton X-100-treated erythrocyte ghosts incubated with intact or rPfSUB1-cleaved rMSP1-Dwt then probed with anti-MSP1 antibodies and 5 nm gold-conjugated secondary antibodies. Error bars, SD.
(F) Overlay assay. Erythrocyte ghosts or Triton X-100-fractionated ghosts were separated by SDS PAGE, transferred to nitrocellulose, then probed with rPfSUB1-cleaved or intact rMSP1-Dwt and binding detected with anti-MSP1 antibodies.
(G) Overlay assay. Erythrocyte ghosts, or purified erythrocyte spectrin (Sigma) mixed with molecular mass marker proteins (GE Healthcare), were subjected to SDS PAGE and either stained or transferred to nitrocellulose and probed as in (F) with rPfSUB1-cleaved rMSP1-Dwt.
(H) Overlay assay. Erythrocyte ghosts probed as in (F) with intact or rPfSUB1-cleaved rMSP1-DCD4wt or rMSP1-DCD4mut. The latter, which is refractory to cleavage in the 38/42 region, did not bind spectrin. See also Figure S4.