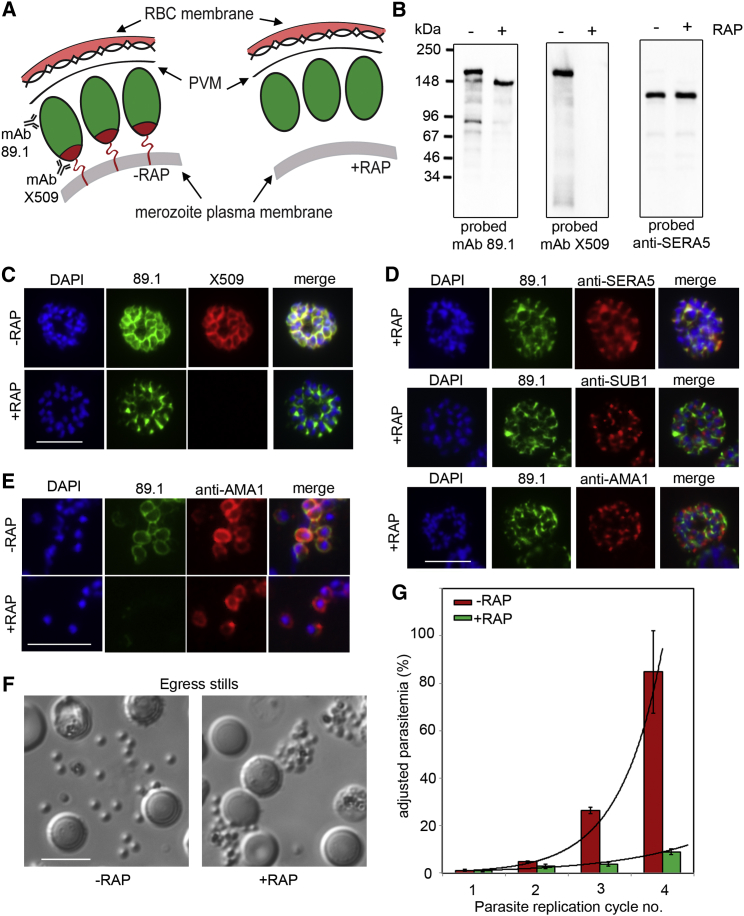
Figure 6.
Truncation of MSP1 Produces an Egress Defect
(A) Predicted RAP-induced MSP1 truncation in the 3D7MSP1flox42C clones, showing loss of the GPI anchor and C-terminal domain containing the mAb X509 epitope.
(B) MSP1 truncation confirmed by western blot of 3D7MSP1flox42C1 clone E3 schizonts, 44 hr following treatment ± RAP. The PV protein SERA5 was used as a loading control.
(C) RAP treatment produces a loss of mAb X509 reactivity and a shift in the IFA pattern of MSP1 to one typical of PV proteins, consistent with the predicted truncation. Numbers of DAPI-stained nuclei did not differ between control and RAP-treated schizonts (mean values: 21.2 ± 3.4 and 20.6 ± 4.0 nuclei per schizont, respectively, n = 24).
(D) IFA showing co-localization of truncated MSP1 with SERA5 indicating a PV location. The punctate localization of SUB1 and the microneme protein AMA1 indicates normal organelle biogenesis.
(E) IFA showing lack of surface-bound MSP1 on merozoites of RAP-treated 3D7MSP1flox42C1 clone E3. Antibodies to AMA1 (which is expressed on free merozoites) were used as a control.
(F) Stills from time-lapse DIC microscopic imaging of egress in control and RAP-treated 3D7MSP1flox42C1 clone E3. Scale bar, 10 μm.
(G) Replication rates of RAP- or control-treated 3D7MSP1flox42C1 clone E3. Cultures were passaged at intervals by 10-fold dilution into fresh medium plus erythrocytes as described in Supplemental Experimental Procedures. Observed parasitaemia values were adjusted for these dilutions and are displayed as adjusted values. The plot shows mean values of three biological replicate experiments. Error bars, SEM. The RAP-treated cultures showed an ∼2.1-fold reduction in replication rate per cycle, but this was an over-estimate of mutant viability due to rapid expansion of the few (∼1%) non-excised parasites in the RAP-treated cultures. See also Figure S6 and Movies S3 and S4.