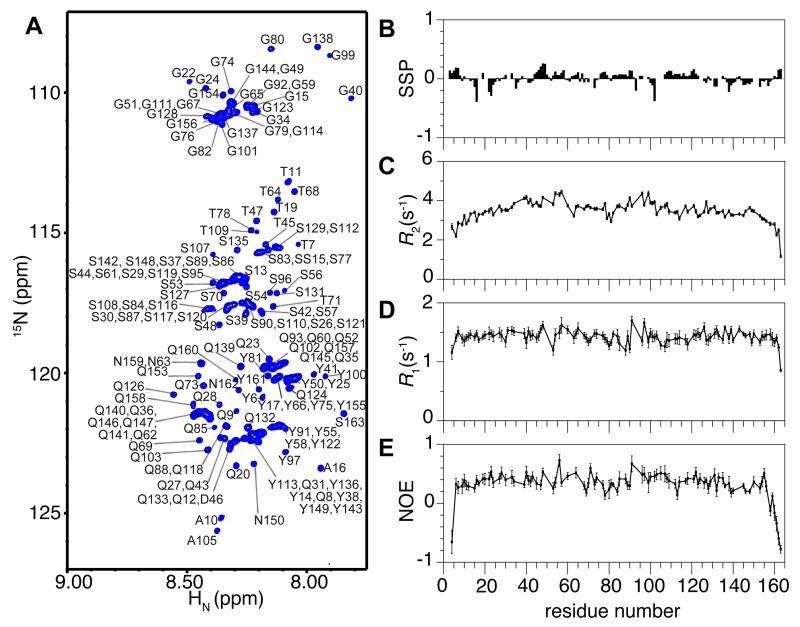
Figure 1.
The FUS low complexity (LC, residues 1-163) domain is disordered as a monomer. (A) NMR spectrum (1H-15N heteronuclear single quantum coherence, HSQC) of the backbone amide region of FUS LC has a narrow chemical shift dispersion indicative of a disordered protein. Residue numbers are labeled (black). (B) Residue-specific secondary structure propensity (SSP) score of FUS LC indicate lack of local secondary structure formation. R2, R1, and (1H)-15N nuclear Overhauser effect (NOE) values (C, D, E, respectively) for the dispersed protein are consistent with disorder across the entire domain. Data are represented as mean +/− st dev. See also Figure S1.