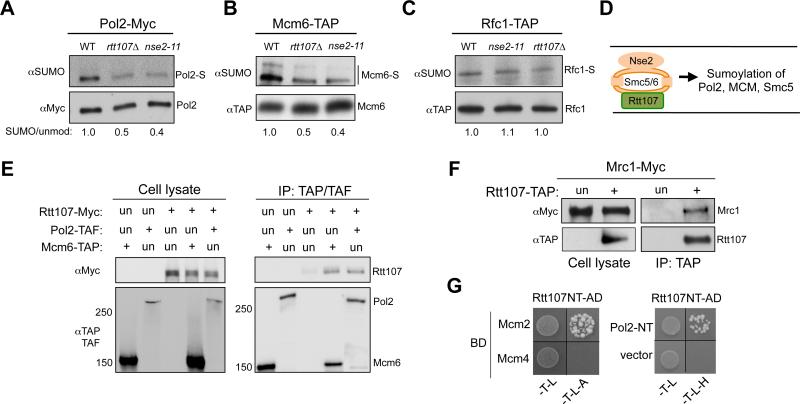
Figure 3. Rtt107 associates with replisome components and affects the sumoylation of Pol2 and Mcm6.
(A-C) Rtt107 and Nse2 mutations reduce the sumoylation levels of specific replisome components. rtt107Δ and nse2-11 reduced the sumoylation levels of Pol2 (A) and Mcm6 (B), but not Rfc1 (C). Experiments were done as in Figure 1F. The relative ratios of sumoylated forms to the unmodified protein forms are indicated.
(D) Schematic of the shared function of Smc5/6 and Rtt107 suggested by this study.
(E-F) Rtt107 associates with replisome components Mcm6, Pol2, and Mrc1. G1 cells were released into medium containing MMS for 90 min. Experimental procedures and labels are the same as those in Figure 1A. (E) Immunoprecipitation of Pol2 or Mcm6 co-purifies Rtt107. (F) Immunoprecipitation of Rtt107 co-purifies Mrc1.
(G) Rtt107 associates with Mcm2 and Pol2 in Y2H assay. Reporter strains contained the DNA binding domain (BD) and DNA activation domain (AD) constructs. Cells were spotted onto SC-Trp-Leu (-T-L) media for plasmid selection and on SC-Trp-Leu-Ade (-T-L-A) or SC –Trp-Leu-His (-T-L-H) media to detect reporter activation. Rtt107 NT: 1-660 amino acids; Pol2 NT: 1-1264 amino acids.