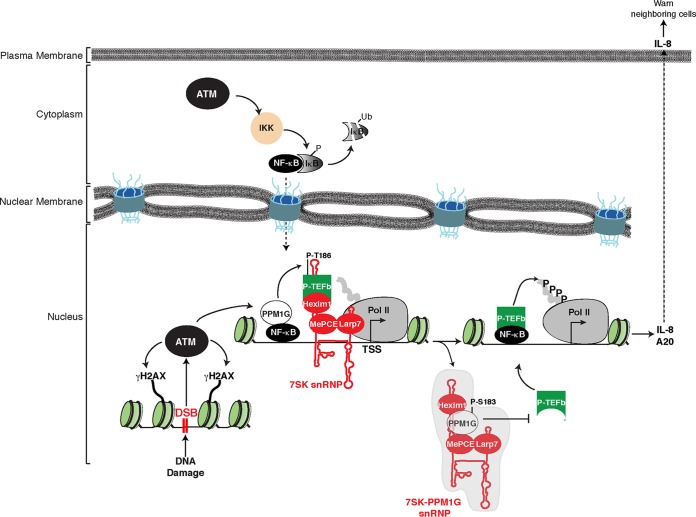
FIG 9.
Model depicting the functional interplay between PPM1G, the 7SK snRNP, and ATM kinase during the activation of the NF-κB transcriptional program in response to DNA damage. In response to double-strand breaks (DSB), ATM activates the NF-κB signaling pathway through phosphorylation of the NEMO subunit of the IKK complex, which in turn phosphorylates the NF-κB inhibitor (IκB), leading to its ubiquitination (Ub) and proteasomal degradation. NF-κB translocates to the nucleus, where it recruits ATM-phosphorylated PPM1G to target genes such as the IL-8 gene, dephosphorylating and releasing the P-TEFb kinase from the promoter-bound 7SK snRNP complex. Upon its release, P-TEFb phosphorylates (P) paused Pol II in proximity to the transcription start site (TSS) to promote transcriptional pause release. After releasing P-TEFb, the inhibitory snRNP subunits are evicted from chromatin, and phosphorylated PPM1G binds 7SK RNA along with Hexim1 to prevent the reassociation of P-TEFb back into the snRNP to sustain transcription elongation. Once the damage is resolved, this regulatory circuitry subsides, PPM1G is dislodged from the snRNP, and P-TEFb is recycled back to promote the formation of the inhibitory 7SK snRNP at the promoter, thereby blocking Pol II pause release.