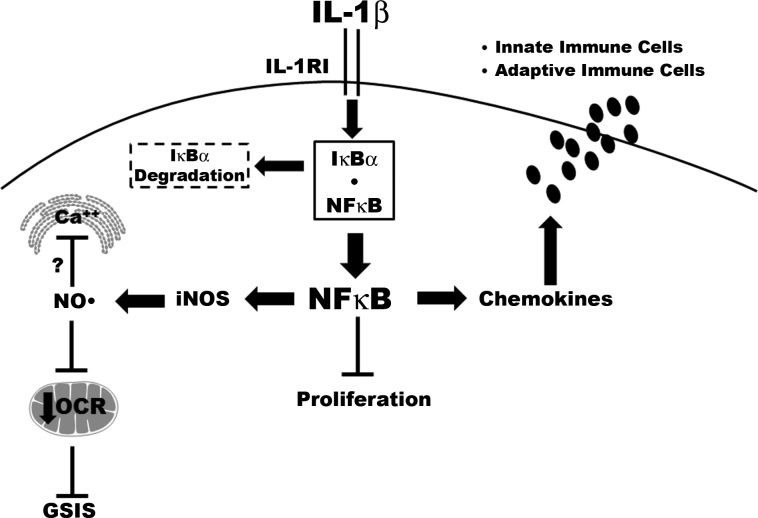
Fig. 8.
NF-κB coordinately regulates chemokine and insulin secretion, with concomitant alterations in proliferation during exposure to IL-1β. IL-1β signals through the IL-1 receptor I (IL-1RI), which promotes the phosphorylation-induced degradation of the IκBα regulatory protein. Degradation of IκBα allows the p65 subunit of NF-κB to move into the nucleus. NF-κB controls the expression of multiple target genes, including iNOS and numerous chemokines. The coordinated changes in gene transcription include increased expression of the iNOS gene, which leads to enhanced production of nitric oxide (NO·). NO· serves as a negative regulator of glucose-stimulated insulin secretion (GSIS). Intracellular calcium is stored in both endoplasmic reticulum (ER) and mitochondria, and the signal-specific release of calcium (e.g., in response to glucose) is potentially inhibited by elevations in intracellular nitric oxide, contributing to diminished insulin secretion. The decrease in cellular OCR is also consistent with the depletion of ATP observed in β-cells exposed to cytokines (17). In addition, IL-1β decreases proliferation in an NF-κB-dependent manner, with concomitant upregulation of chemokine synthesis and secretion. Increasing the amount of chemokines released from β-cells influences both innate and adaptive immune responses. Unlike insulin secretion, β-cell chemokine secretion is independent of cellular nitric oxide levels.