Abstract
Perinatal asphyxia and aerodigestive symptoms are troublesome. We tested the hypothesis that pharyngeal provocation alters proximal and distal aerodigestive reflex coordination and kinetics in infants with hypoxic ischemic encephalopathy (HIE), compared with healthy controls. Specifically, we characterized the sensory-motor properties of pharyngeal provocation-induced effects on upper esophageal sphincter (UES) and lower esophageal sphincter (LES) reflexes. Ten orally fed controls (32.0 ± 1.5 wk gestation) and 25 infants with HIE (38.1 ± 0.4 wk gestation) were evaluated at 39.7 ± 0.9 and 41.9 ± 0.6 wk postmenstrual age respectively. Pharyngo-esophageal reflexes evoked upon graded water stimuli were tested using water-perfusion micromanometry methods. Analysis included sensory-motor characteristics of pharyngeal reflexive swallow (PRS), pharyngo-UES-contractile reflex (PUCR), esophageal body-waveform kinetics, and pharyngo-LES-relaxation reflex (PLESRR). For controls vs. infants with HIE, median appearance, pulse, grimace, activity, respiration (APGAR) scores were 6 vs. 1 at 1 min (P < 0.001) and 8 vs. 3 at 5 min (P < 0.001). Upon pharyngeal- stimulation, HIE infants (vs. controls) had frequent PUCR (P = 0.01); increased UES basal tone (P = 0.03); decreased LES basal tone (P = 0.002); increased pharyngeal-waveforms per stimulus (P = 0.03); decreased frequency of LES relaxation (P = 0.003); and decreased proximal esophageal contractile amplitude (P = 0.002), with prolonged proximal esophageal contractile duration (P = 0.008). Increased tonicity and reactivity of the UES and dysregulation of LES may provide the pathophysiological basis for pooling of secretions, improper bolus clearance, and aspiration risk. Deficits in function at the nuclear or supranuclear level involving glossopharyngeal and vagal neural networks and respiratory regulatory pathways involved with aerodigestive protection may be contributory.
Keywords: neonate, pharyngo-esophageal motility, aerodigestive sphincters, hypoxic ischemic encephalopathy
birth asphyxia occurs in 1–4 per 1,000 live births in the United States and other developed countries and in 1–9 per 1,000 live births in underresourced countries, often manifesting as hypoxic ischemic encephalopathy (HIE) (2, 28, 31). HIE presents with a multitude of airway and digestive symptoms; as such, aerodigestive management of these patients can be challenging. Specifically, swallowing reflexes are needed to handle oral secretions, gastroesophageal reflux (GER), as well as during postemetic throat clearance or posttussive states; such troublesome symptoms pose a constant challenge to parents and health care providers. As survival with morbidity is increasing among these prototypes of HIE, the consequences of dysphagia, gastroesophageal reflux disease, and aspiration syndromes are also increasing (6, 21, 27). Gastrostomy tube feeding, a common outcome in infants with severe HIE, is associated with an increased risk of aspiration (23). While some neonatal centers in developed and developing countries may discharge infants with feeding tubes, many countries, including the United States, continue to require competent feeding by breast or bottle as a prerequisite for hospital discharge (1). As such, delays in the ability to achieve the oral feeding milestone can significantly prolong the length of stay for affected infants.
Pharyngeal provocation can happen during oral bolus transit, expectoration, or proximal ascent of refluxate or emesis. In the absence of timely activation of aerodigestive reflexes, infants are at risk for airway compromise including recurrence of asphyxiating events, aspiration pneumonia, and persistent feeding difficulties. In addition, potential recurring asphyxiating events can occur that require interventions such as suctioning of aerodigestive tract, supplemental oxygen, airway resuscitation, or sepsis work-up (12). The neurosensory and neuromotor mechanisms behind infant adaptation to provocative events are not well understood in this developmentally vulnerable cohort. Recently, we demonstrated that healthy neonates respond to pharyngeal stimulus more frequently with pharyngeal reflexive swallows (PRS) so as to clear the bolus, as opposed to pharyngo-upper esophageal sphincter contractile reflexes (PUCR) (12). Additionally, healthy neonates have intact function of the lower esophageal sphincter (LES), including relaxation during spontaneous swallowing as well as during pharyngeal provocation-induced adaptive swallowing, thereby facilitating bolus transit (24–26). However, the relationship between pharyngeal stimulation and adaptive aerodigestive function in infants with HIE is unclear. Infants with HIE have feeding difficulties manifesting as oropharyngeal inertia, pooling of secretions, delayed clearance of feeds, and inadequate airway protection. Whether this is due to sensory or motor malfunctions (or both) is unclear. Therefore, our aims were to characterize pharyngeal stimulation-induced adaptive responses in infants with HIE and compare these responses with those of healthy controls. The rationale for our hypothesis was infant hypoxic ischemic injury may affect both afferent and efferent nerve impulse transmission, leading to both skeletal and smooth muscle dysfunction, which may result in pooling of oro-pharyngeal secretions. Specifically, we tested the hypothesis that infants with HIE have altered response sensitivity and magnitude of aerodigestive reflexes and coordination manifesting as altered initial response, variation in response duration, terminal swallow characteristics and respiratory adaptation. Specifically, we examined PRS, PUCR, and pharyngo-LES relaxation reflex (PLESRR).
MATERIALS AND METHODS
A total of 35 pharyngo-esophageal manometry studies were performed. No adverse cardio-respiratory events were noted during any of these studies. Infants were included in the HIE group if they were >35 wk gestational age (GA), experienced acute perinatal asphyxia defined by an acute perinatal event (placental abruption, cord prolapse, abnormal fetal heart rate), and presented with signs of encephalopathy as per Sarnat staging (32). The control group included infants >31.0 wk GA with safe oral feeding skills at study, who did not have any congenital, genetic, or chromosomal abnormalities. Practical and ethical obstacles impede recruitment of healthy age-matched full-term controls. Since the control infants were studied at term equivalent postmenstrual age (PMA), they are maturationally appropriate for comparison, as they had acquired oral feeding skills comparable to term infants by the time of study (16). Protocol approval was obtained from the Institutional Research Review Board (IRB) at the Nationwide Children's Hospital Research Institute before initiation of this project. The study protocol conforms to the guidelines of the IRB policy and the Health Insurance Portability and Accountability Acts (HIPAA). Informed consents and HIPAA authorization were obtained from parents before the study. Enrollment and studies were performed consecutively, based on the inclusion and exclusion criteria, as well as scientific appropriateness, and parental consenting. Parents were not compensated for allowing infants to participate in the study. As infants with HIE receive an MRI as standard of care, this information was obtained before hospital discharge. Feeding method at 1 yr of age was obtained from electronic medical record.
Manometry methods.
Multimodal pharyngeal provocations, using graded volumes of sterile water, were given and manometric recordings were analyzed as previously described (10–15). Briefly, the catheter assembly (Dentsleeve International; Mui Scientific, Ontario, Canada) was connected to the pneumohydraulic micromanometric water perfusion system via resistors, pressure transducers (TNF-R disposable pressure transducers), and amplifiers (Solar modules, Solar 2; MMS Medical Instruments, Dover, NH). A 6-French manometry catheter assembly with dual sleeves [positioned at the upper esophageal sphincter (UES) and LES], four side-ports recording from the pharynx, the proximal-, middle-, and distal esophageal loci, in addition to a terminal gastric recording port was used. The micromanometric water perfusion rates were 0.02 ml·min−1·port−1 for esophageal ports, 0.01 ml·min−1·port−1 for the pharyngeal port, and 0.04 ml·min−1·port−1 for the sleeves. The catheter was zeroed at the level of the subject's esophagus and then passed nasally while the infant lay supine and unsedated. The manometry catheter was properly positioned using the pull through technique to identify high-pressure zones and was well secured.
Manometric experimental protocol.
After ∼15 min of adaptation upon catheter placement, upstream and downstream responses to increasing graded volumes of pharyngeal infusions (sterile water stimuli, 0.1, 0.3, and 0.5 ml) were evaluated. The rationale for using graded volumes of water was to determine the threshold sensitivity and response latency to abrupt liquid stimuli (34). The rationale for giving infusions in this manner was to simulate an abrupt natural pharyngeal bolus provocation and to test volume dose-response relationships. Abrupt provocations happen naturally such as in the event of gastroesophageal reflux, during the posttussive state or during bolus entry into the pharynx. The specific responses of interest PRS, PUCR, esophageal body contraction amplitude, duration, velocity, and PLESRR. Proximal and distal sphincter resting tone and presence of polymorphic waveforms (PMWs) were also evaluated. Respiratory waveform characteristics were monitored using respiratory inductance plethysmography methods. Subject safety was also monitored by direct nursing observation.
Manometry data analysis.
Analytical methods for defining the manometry characteristics have been described before (5, 9–15, 26) and are briefly defined as follows: 1) pharyngeal reflexive swallow (PRS) was defined as a deglutition response to pharyngeal stimulation; onset is indicated by the presence of a pharyngeal waveform in association with UES relaxation, followed by propagation into the proximal, middle, and distal esophageal segments and further accompanied by LES relaxation; 2) pharyngo-upper esophageal sphincter contractile reflex (PUCR) was defined as an increase of ≥4 mmHg above UES resting tone in response to a pharyngeal stimulus; 3) pharyngo-lower esophageal sphincter relaxation reflex (PLESRR) was defined as LES relaxation of at least 5 mmHg below LES resting pressure after stimulus onset; 4) response latency to PRS was defined as the duration from the onset of the stimulus to the onset of the pharyngeal contraction; 5) response latency to PUCR was defined as the duration from the onset of stimulus to an increase in the UES of at least 4 mmHg; 6) LES nadir duration was defined as the onset time from which the LES relaxes to 5.0 mmHg, across the lowest LES pressure, to the offset time until it recovers to 5.0 mmHg; 7) duration of respiratory change was defined as modification of normal respiration in the thoracic plethysmography channel from the onset of the infusion until normal respiration patterns were restored; 8) polymorphic waveforms (PMWs) were defined as multiple esophageal body waveforms noted in the proximal, middle, or distal esophageal body. UES and LES resting pressures were evaluated before infusion onset, relative to atmospheric or gastric pressure, respectively, at end expiration. All manometric patterns and reflexes were visually identified at the time of study, and coinvestigators were trained to recognize them during subsequent waveform analysis. Our analysis was conducted based on previously validated and published methods and definitions to which we strictly adhered. Interrater/intrarater variability was avoided by agreement of at least two investigators on recorded analysis. Training and retraining of personnel has been completed during periodic training sessions as conducted by the principal investigator (S. R. Jadcherla). Because of strict adherence to a priori definitions and agreement by at least two, if not three, coinvestigators, our agreement rates are close to perfection.
Statistical analysis.
Comparisons of demographic characteristics between HIE and control groups were performed using unpaired t-tests for continuous variables or χ2-square tests for categorical variables. Wilcoxon rank-sum tests were used if the continuous variable was not normally distributed. Due to the presence of repeated measures within each subject, manometric data were analyzed using linear mixed models. Generalized estimating equation (GEE) models, which are an extension of generalized linear models, were chosen for categorical outcomes. Linear mixed and GEE models were performed with and without controlling for the effects of GA and chronological age (CA), so as to mitigate the discrepancies in GA and CA between HIE and control groups. GA- and CA-matched healthy controls would have been ideal, but ethical reasons preclude us from such clinical study designs. Since both groups were similar with respect to PMA, we did not control for its effects. Pearson's correlation was used to test the correlation among GA, CA, and PMA. GA was found to be significantly correlated with PMA (r = 0.6, P = 0.0003) and CA (r = −0.7, P < 0.0001). Data are presented as means ± SE, estimated means ± SE, percentage, median, or as otherwise indicated. P < 0.05 was considered significant. Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Responses to a total of 240 sterile water infusions administered into the pharynx were analyzed (n = 69 infusions in controls; n = 171 infusions in HIE infants) from 35 patients (n = 10 control; n = 25 HIE). Clinical characteristics for 10 control patients (4 males; 32.0 ± 1.5 wk GA) and 25 HIE patients (11 males; 38.0 ± 0.4 wk GA; P = 0.003 for GA comparison; 7.8 ± 1.5 vs. 3.8 ± 0.4 wk CA; P = 0.03) are outlined in Table 1. In control infants, 2 of 10 infants (20%) were discharged with gastrostomy tubes. These infants had oral skills at the time of study and discharge. In infants with HIE, 13 of 24 (54%) had gastrostomy tubes placed at discharge (P = 0.1 vs. control infants). Infants were discharged with G-tube placement for supplemental nutritional intake to avoid failure to thrive. This was a clinical decision made by the health care providers. One infant with HIE did not have discharge feeding method documented.
Table 1.
Demographic characteristics
| Control (n = 10) | HIE (n = 25) | P Value | |
|---|---|---|---|
| 1-min APGAR | 6.0 (4.0–8.0) | 1.0 (0.0–2.0) | <0.001 |
| 5-min APGAR | 8.0 (8.0–9.0) | 3.0 (0.0–5.0) | <0.001 |
| PMA at study, wk | 39.7 ± 0.9 | 41.9 ± 0.6 | 0.05 |
| Weight at study, kg | 3.2 ± 0.2 | 3.8 ± 0.2 | 0.05 |
| FOC at study, cm | 35 (32–37) | 35 (33.5–36) | 0.8 |
| Exclusive oral feeds at study (no.), % | 7 (70) | 2 (8) | 0.002 |
| Exclusive oral feeds at discharge (no.), % | 8 (80) | 11 (44) | 0.2 |
Data are presented means ± SE, median (range), and as percent; n = 24 total infants with hypoxic ischemic encephalopathy (HIE) that had discharge feeding outcomes available.
APGAR, appearance, pulse, grimace, activity, respiration; PMA, postmenstrual age; FOC, fronto-occipital circumference. Italicized values indicate significant P values.
In infants with HIE, per MRI during hospitalization, white matter changes were evident in n = 10 (40%), grey matter changes were evident in n = 6 (24%), and cerebellar injury was evident in n = 17 (68%). At 1 yr of age, one control infant was lost to follow-up; the remaining nine control infants had documented survival; eight out of these nine (89%) control infants had additional growth and neurodevelopmental follow-up outcomes recorded. Of these infants, six of eight (75%) were exclusively oral feeding at 1 yr of age. For infants with HIE, 100% had documented survival at 1 yr age, and 12 of 21 (57%) infants were exclusively oral feeding at 1 yr of age (P = 0.009 vs. control infants). Of these infants, 23 had additional neurodevelopmental outcomes recorded. No control infants required respiratory support at 1 yr; however, five infants with HIE required respiratory support (n = 4 tracheostomy; n = 1 nasal cannula). No control infants were given a diagnosis of cerebral palsy, hearing loss, or vision problems at 1 yr of age; however, cerebral palsy was diagnosed in 52% (12 out of 23), hearing loss in 26% (6 out of 23), and vision problems in 17.4% (4 out of 23) infants with HIE.
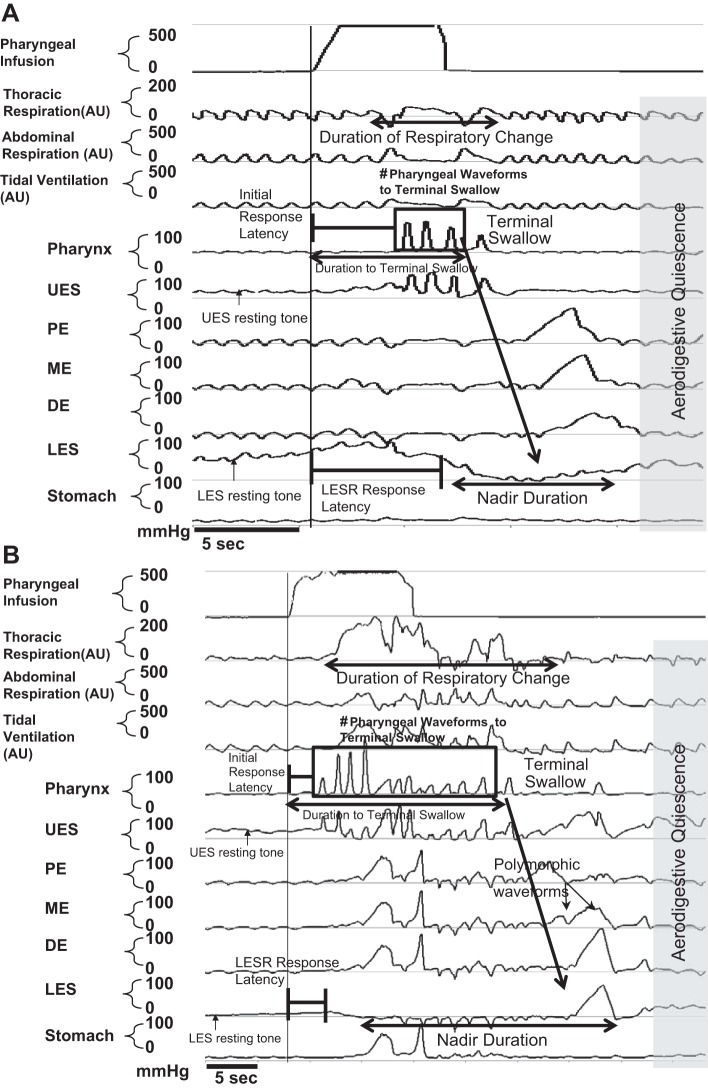
An example of pharyngeal infusion-induced esophageal manometry changes in both control infants and infants with HIE is shown in Fig. 1, A and B. We analyzed UES and LES resting tone, cumulative aerodigestive response duration, number of pharyngeal peaks to the terminal swallow that resulted in restoration of aerodigestive quiescence, presence of PUCR, and response latency to PUCR or PRS, duration of LES nadir and respiratory perturbations, and esophageal body characteristics of the terminal swallow.
Fig. 1.
Effect of pharyngeal stimulation on aerodigestive sensory motor characteristics: control infant response to 0.3-ml pharyngeal water infusion (A) and hypoxic ischemic encephalopathy (HIE) infant response to 0.3-ml pharyngeal water infusion (B). Manometry data were analyzed as labeled. Note the prolonged response in HIE infants. AU, arbitrary units; UES, upper esophageal sphincter; PE, proximal esophagus; ME, mid-esophagus; DE, distal esophagus; LES, lower esophageal sphincter.
Neurosensory and neuromotor characteristics of proximal aerodigestive interactions.
Resting UES pressure in control infants was 11.0 ± 2.2 vs. 16.8 ± 1.5 mmHg in infants with HIE (P = 0.03). In response to pharyngeal stimulation, the proportions of PUCR: PRS were compared between the groups (Fig. 2A). The frequency of recruitment of pharyngeal peaks with each stimulus were also compared (Fig. 2B). Sensory-motor characteristics of esophageal and respiratory adaptive responses to pharyngeal stimulation were compared, with reference to PUCR and PRS response latency, duration of pharyngo-esophageal motor activity, and respiratory change (Fig. 2C).
Fig. 2.
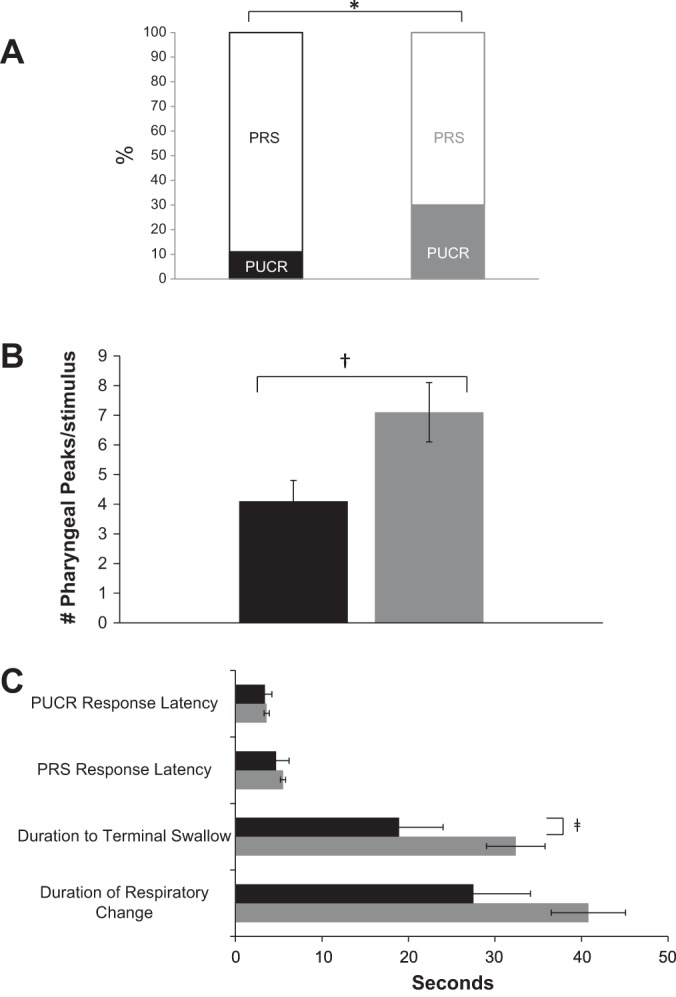
Initial response characteristics and subsequent response duration (black bars: control; gray bars: HIE; *P = 0.01; †P = 0.02; ‡P < 0.05). Initial response distribution (A); pharyngeal reflexive swallow (PRS) recruitment terminal swallow (B); and initial response latency (seconds) and duration of response (seconds) (C) defined as follows: response latency to pharyngo-UES-contractile reflex (PUCR): the duration from the onset of stimulus to an increase in the UES of at least 4 mmHg; response latency to PRS: the duration from the onset of the stimulus to the onset of the pharyngeal contraction; duration to terminal swallow: the time from the onset of pharyngeal stimulus to the onset of the pharyngeal waveform of the terminal swallow; and duration of respiratory change: time from the initial modification of normal respiration in the thoracic plethysmography channel from the onset of the infusion until normal respiration patterns were restored; note the prolonged duration until the terminal swallow in infants with HIE. Also note the prolonged duration of respiratory change in infants with HIE, although not statistically significant (P = 0.1).
Stimulus-volume response relationships were compared for number of pharyngeal peaks with each infusion per volume for control vs. HIE infants until restoration of aerodigestive quiescence (Fig. 3A). The relationship between duration of respiratory change and latency until the terminal swallow for control vs. HIE infants is shown (Fig. 3B). The Spearman correlation coefficient for the data set is indicated in Fig. 3; with the indicated outliers removed, the correlation remains positive (R = 0.95 and R = 0.86 for control infants and HIE infants, respectively).
Fig. 3.
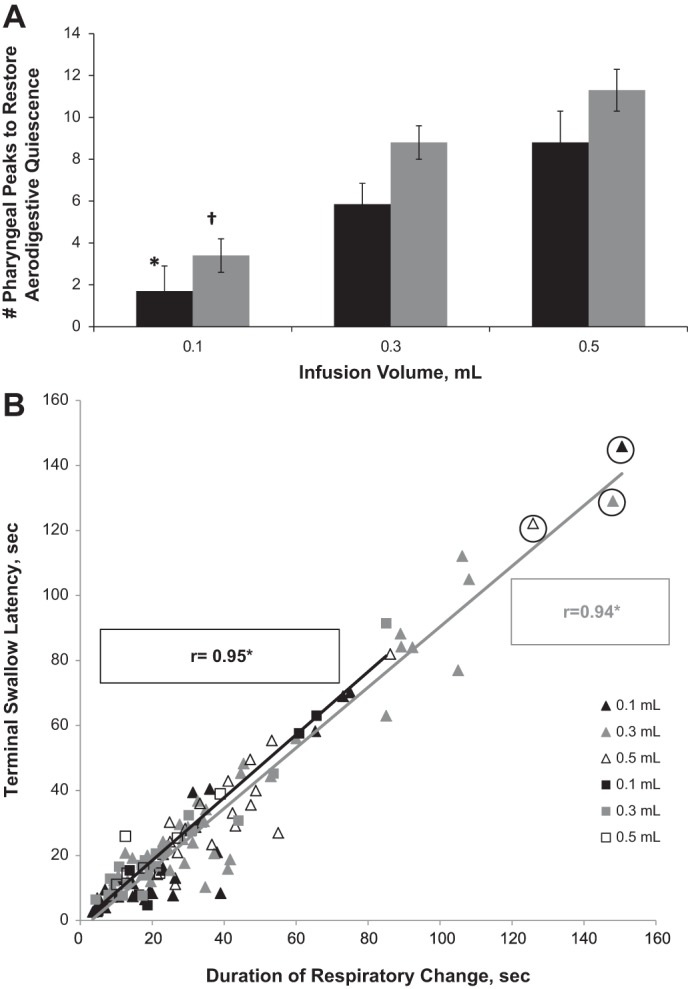
Total response duration and characteristics. A: infusion volume (black bars: control; gray bars: HIE). *P < 0.05 for volumetric effect compared with 0.5 ml; †P < 0.05 for volumetric effect compared with 0.3- and 0.5-ml infusions; volumetric PRS recruitment to aerodigestive quiescence. Note HIE patients have a significant volumetric response for 0.1-ml infusions compared with 0.3- and 0.5-ml infusions. Control infants have a significant volumetric response between 0.1-ml infusions compared with 0.5-ml infusions. B: durations of respiratory change (open squares: control; open triangles: HIE). *P < 0.0001, respiratory change duration is highly correlated with terminal swallow latency in HIE infants.
Characteristics of esophageal body propulsion.
Esophageal body contraction amplitude and duration with regard to terminal swallowing are shown in Table 2. With each pharyngeal stimulus, the number of polymorphic esophageal body waveforms in controls vs. HIE was 1.0 ± 0.1 vs. 1.2 ± 0.04 (P = 0.01). The duration (seconds) of polymorphic waveform activity in controls vs. HIE was 3.2 ± 0.8 vs. 5.5 ± 0.4 (P = 0.02). Peristaltic velocity (cm/s) in controls vs. HIE from the proximal to distal esophagus was 4.2 ± 1.0 vs. 2.1 ± 0.7 (P = 0.09). The rate of esophageal body contraction (mmHg/s) was for the proximal esophagus 52.4 ± 7.9 vs. 24.8 ± 5.1 (P = 0.005); for the mid-esophagus 48.2 ± 4.5 vs. 43.0 ± 2.8 (P = 0.3); and for the distal esophagus 29.6 ± 2.2 vs. 31.9 ± 3.1 (P = 0.6) for controls vs. HIE infants, respectively.
Table 2.
Characteristics of esophageal body propagation kinetics
| Control (n = 10) | HIE (n = 25) | P Value | |
|---|---|---|---|
| Proximal esophagus | |||
| Contraction amplitude, mmHg | 50.1 ± 3.8 | 35.3 ± 2.5 | 0.002 |
| Contraction onset to peak amplitude*†, s | 1.5 ± 0.3 | 1.9 ± 0.2 | 0.2 |
| Peak amplitude to contraction offset*†, s | 1.5 ± 0.2 | 2.2 ± 0.1 | 0.008 |
| Mid-esophagus | |||
| Contraction amplitude, mmHg | 67.6 ± 7.0 | 74.1 ± 4.3 | 0.4 |
| Contraction onset to peak amplitude*, s | 1.5 ± 0.2 | 2.0 ± 0.1 | 0.03 |
| Peak amplitude to contraction offset, s | 1.9 ± 0.2 | 2.4 ± 0.1 | 0.06 |
| Distal esophagus | |||
| Contraction amplitude, mmHg | 55.6 ± 5.4 | 56.3 ± 3.7 | 0.9 |
| Contraction onset to peak amplitude*, s | 2.1 ± 0.2 | 2.2 ± 0.1 | 0.8 |
| Peak amplitude to contraction offset*, s | 1.9 ± 0.3 | 2.4 ± 0.2 | 0.1 |
Data are presented as estimated means ± SE; P < 0.05 is considered significant.
P < 0.05, when controlling for gestational age;
P < 0.05, when controlling for chronological age. Italicized values indicate significant P values.
Effects of pharyngeal provocation on the LES.
Resting LES tone was 26.0 ± 2.5 mmHg in control infants vs. 16.4 ± 1.6 mmHg in infants with HIE (P = 0.002). The frequency of PLESRR in control vs. HIE infants was 79 vs. 57% (P = 0.003). The effects of pharyngeal stimulus on LES nadir pressure and duration are shown (Fig. 4A). Stimulus-volume response relationships were compared for LES nadir duration (Fig. 4B).
Fig. 4.
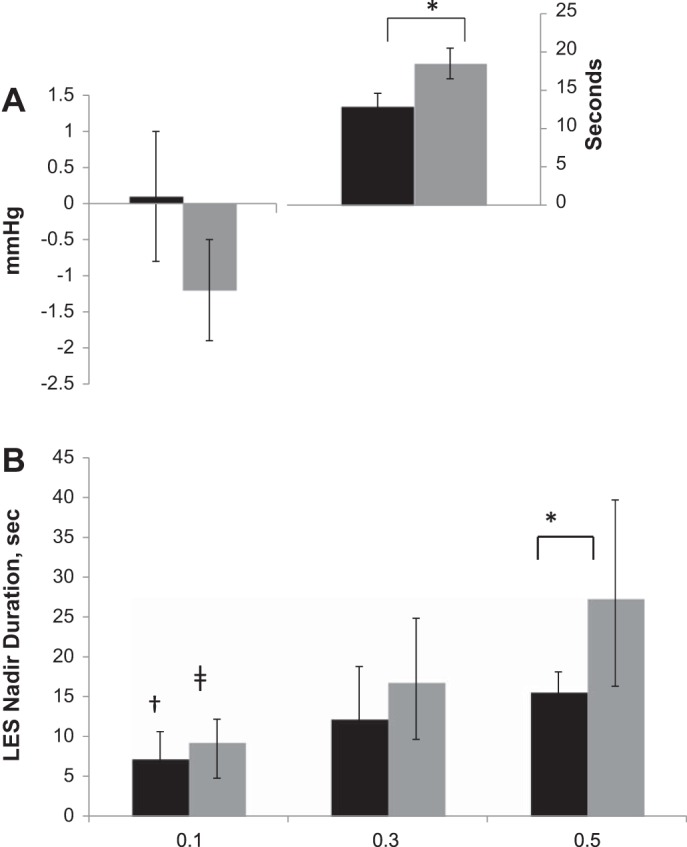
LES characteristics (black bars: control; gray bars: HIE; *P < 0.05; †P < 0.05 for volumetric effect compared with 0.5 ml; ‡P < 0.05 for volumetric effect compared with 0.3- and 0.5-ml infusions). A: LES nadir pressure (left) and LES nadir duration (right); P = 0.3 for comparison of LES nadir pressure. B: volumetric LES relaxation reflex recruitment relationships.
DISCUSSION
Using an experimental design and provocative interrogation of pharyngo-esophageal motility reflexes, we investigated the mechanism of pharyngeal adaptation in infants with HIE compared with neurologically intact controls. As infants with HIE have frequent aerodigestive problems and difficulty achieving feeding milestones, our working hypothesis was that HIE modifies central swallowing pattern generation and adaptive pharyngo-esophageal reflexes. In the current study, we demonstrated the neurosensory and neuromotor characteristics of significant pharyngeal maladaptation in infants with HIE. Importantly, central swallowing generation, swallowing frequency, and duration, in addition to UES and LES tone and reactivity during pharyngeal provocation were all noted to be aberrant. Specifically the cardinal findings of our study indicate that compared with control infants, infants with HIE have the following: 1) increased resting UES tone; 2) increased frequency of PUCR in response to pharyngeal stimulation; 3) increased recruitment of pharyngeal peaks per stimulus; 4) increased duration to restoration of aerodigestive quiescence (until terminal swallow); 5) increased presence and duration of polymorphic waveforms; 6) decreased contraction amplitude and increased duration from the peak to offset of contraction in the proximal esophagus; 7) increased contraction duration in the mid-esophagus; 8) decreased resting LES tone; 9) decreased frequency of PLESRR; and 10) increased LES nadir duration.
Infants with HIE clearly had more difficulty with feeding, clearance of oral secretions, maintenance of airway and aerodigestive coordination as evidenced by the significant number needing gastrostomy, tracheostomy, and supportive oxygen therapy. Prolonged and infrequent adaptive responses to pharyngeal stimuli are suggestive of delays in conductivity of afferent and/or efferent neural communications between the point of stimulation and point of motor responsiveness. Aerodigestive symptoms in HIE, such as pooling of oral secretions, may be related to altered neurosensory mechanisms that fail to evoke swift clearance of secretions away from the oral cavity. It may be noted that although the HIE group did maintain sensitivity of upper aerodigestive tract reflex recruitment, the motor responses were uncoordinated and prolonged; however, sensitivity of reflex recruitment in the lower aerodigestive tract was decreased.
Pharyngo-UES interactions upon pharyngeal provocation in healthy preterm infants have been characterized by us as well as others (12, 15, 18, 29). Specifically, in healthy neonates, PRS occurs more frequently than PUCR in response to pharyngeal provocation (12). In addition, PLESRR is activated during pharyngeal reflexive swallowing (22) and becomes robust with maturation (15, 26). On the other hand, in infants with HIE, esophageal mechano-distension results in upregulation of central vagal (cholinergic) effects manifesting as increased excitation demonstrated by exaggerated magnitude of UES contractile reflex or increased inhibition as evidenced by exaggerated LES relaxation reflex pressure and duration (6, 27, 33). Similarly, our current study data demonstrate modified and possibly increased cholinergic neural activity as the basis for increased UES resting pressure and more frequent PUCR responses. Decreased contraction amplitude coupled with decreased rate of muscle contraction and prolonged duration of waveforms or polymorphic waveforms in the skeletal muscle part of the proximal esophagus may be due to phenomenon of summation of contractions akin to tetanic contractions (3). Alternatively, animal experimental data reveal that antecedent hypoxic exposure can result in a decrease in end-plate potential to below firing threshold, which could contribute to modified muscle function in infants with HIE, as the release of acetylcholine at the neuromuscular junction would be blocked (7).
Infants with HIE also experienced an increased number of adaptive pharyngeal waveforms, prolonged contraction and relaxation in the mid esophagus, and prolonged duration to reach terminal swallow restoring respiratory quiescence vs. control infants. Heightened responses to graded infusion volumes in HIE compared with controls suggests preserved volume sensitivity in HIE infants. HIE infants experience prolonged respiratory changes which are proportional to the time taken for bolus clearance. Altered sensory-motor characteristics of pharyngo-UES interactions and prolonged respiratory changes in the HIE group, when compared with control infants, suggest dysfunctional interactions between neural pathways and circuitry related to swallowing and breathing. These observations are supported by animal experiments demonstrating that exposure to acute intermittent hypoxia leads to synaptic inhibition when norepinephrine secretion is increased within the pre-Bötzinger complex, which is an area critical for breathing (37).
Supranuclear or nuclear level lesions in HIE infants may modify functioning of the vagus nerve and respiratory neural pathways involved with swallowing, respiration, and aerodigestive protection. HIE infants are noted to have decreased basal LES tone, decreased frequency recruitment of PLESRR, and volume-dependent prolonged nadir duration. Decreased LES resting tone is likely be due to an imbalance between excitatory and inhibitory vagal tone. Prolonged PLESRR nadir duration in infants with HIE may be due to sustained inhibitory input to the LES, implicating dysregulation in restoration of LES tone, while decreased frequency of PLESRR may be due to impaired sensitivity. Impaired sensory-motor responses may indicate neurosensory and neuromotor malfunctions of cranial nerves IX and X, which regulate and modulate pharyngeal swallowing reflexes and LES kinetics. This can be explained by sustained release of inducible nitric oxide during the reperfusion phase of HIE (4, 8, 36). Additionally, the effect of hypoxia on respiratory neural output may be linked with modulation of LES tone (17).
Recent research has demonstrated feeding and communication impairment are noted among HIE survivors with grey matter lesions (20, 21). Additionally, severity of white matter injury is correlated with poor neurodevelopmental outcomes at age 2 (19). In the current study, the instance of white matter changes were more frequent in the studied population (40%) than grey matter changes (24%), and cerebellar abnormalities were noted most frequently (68%) in infants with HIE. The cerebellum is an important center for regulation and coordination of motor tone (30). Abnormal motor function, such as in the current study, may be due to such lesions. It is also interesting to note that about half of HIE infants were on exclusive oral feeds at discharge and remained to be on exclusive oral feeds at 1 yr of age. This indicates that despite neurological injury, neuroplasticity may allow for the fast developing brain of an infant to adapt to injury to allow for organism survival. Additionally, radiological markers of neurological injury do not necessarily correlate with functional feeding milestones.
As is the case with any clinical study, the current study has limitations. The oral pharyngeal phase of swallowing has not been included as part of this investigation. Further work in this area is necessary. Additionally, information regarding maturation of oral feeding skills in infants with HIE is lacking. Further studies are necessary to address intervention for such infants during interval follow-up evaluation.
The implications of this study are several. It is commonly speculated that HIE infants have varying degrees of upper and lower aerodigestive maladaptation, which may lead to aspiration syndromes (35). The current study supports such concerns, compared with control infants, infants with HIE had aberrant UES function, prolonged adaptive responses to pharyngeal stimulation, and decreased LES basal tone. Implementation of various medical and surgical therapies intended to address these issues is delayed due to lack of information on pathophysiological changes in a growing and developing infant with HIE. Our findings may lend support to personalization of therapies based on specific mechanistic diagnosis. In conclusion, infants with HIE exhibited increased tonicity and reactivity of the UES in addition to dysregulation at LES. These findings may explain the pathophysiological basis for pooling of secretions, improper bolus clearance, and aspiration risk. These conclusions implicate the presence of lesions at the nuclear or supranuclear level involving glossopharyngeal and vagal neural networks and respiratory regulatory pathways that are involved with swallowing and airway protection as the basis for dysfunction.
GRANTS
This study has been supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant 2RO1-DK-068158 (to S. R. Jadcherla).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.K.G., L.W., and S.R.J. conception and design of research; I.K.G., T.R.S., S.S., L.W., and S.R.J. analyzed data; I.K.G., T.R.S., and S.R.J. interpreted results of experiments; I.K.G., T.R.S., S.S., and S.R.J. drafted manuscript; I.K.G., T.R.S., S.S., L.W., and S.R.J. edited and revised manuscript; I.K.G., T.R.S., S.S., L.W., and S.R.J. approved final version of manuscript; T.R.S. prepared figures; S.R.J. performed experiments.
REFERENCES
- 1.American Academy of Pediatrics Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate. Pediatrics 122: 1119–1126, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Ballot D. Cooling for newborns with hypoxic ischaemic encephalopathy: RHL commentary (last revised: 1 October 2010). In: The WHO Reproductive Health Library. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 3.Barrett KB, Barman SM. Ganong's Review of Medical Physiology. New York: McGraw-Hill, 2009. [Google Scholar]
- 4.Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 76: 126–152, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Gulati P, Kim W, Fernandez S, Shaker R, Jadcherla SR. Effect of postnatal maturation on the mechanisms of esophageal propulsion in preterm human neonates: primary and secondary peristalsis. Am J Gastroenterol 104: 411–419, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill CD, Jadcherla SR. Esophageal mechanosensitive mechanisms are impaired in neonates with hypoxic-ischemic encephalopathy. J Pediatr 162: 976–982, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbard JI, Loyning Y. The effects of hypoxia on neuromuscular transmission in a mammalian preparation. J Physiol 185: 205–223, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest 111: 163–169, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadcherla SR, Chan CY, Fernandez S, Splaingard M. Maturation of upstream and downstream esophageal reflexes in human premature neonates: the role of sleep and awake states. Am J Physiol Gastrointest Liver Physiol 305: G649–G658, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr 143: 31–38, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Jadcherla SR, Duong HQ, Hofmann C, Hoffmann R, Shaker R. Characteristics of upper oesophageal sphincter and oesophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil 17: 663–670, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr 151: 597–603, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. Am J Gastroenterol 104: 2572–2582, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr 149: 77–82, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadcherla SR, Shubert TR, Gulati IK, Jensen PS, Wei L, Shaker R. Upper and lower esophageal sphincter kinetics are modified during maturation: effect of pharyngeal stimulus in premature infants. Pediatr Res 77: 99–106, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and co-morbidities on feeding milestones in neonates: a retrospective study. J Perinatol 30: 201–208, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiatchoosakun P, Dreshaj IA, Abu-Shaweesh JM, Haxhiu MA, Martin RJ. Effects of hypoxia on respiratory neural output and lower esophageal sphincter pressure in piglets. Pediatr Res 52: 50–55, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr 92: 721–727, 2003. [PubMed] [Google Scholar]
- 19.Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, Cowan FM. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr 161: 799–807, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, Lawrence S, Aloysius A, Rutherford MA, Cowan FM. Feeding and communication impairments in infants with central grey matter lesions following perinatal hypoxic-ischaemic injury. Eur J Paediatr Neurol 16: 688–696, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Biarge M, Diez-Sebastian J, Rutherford MA, Cowan FM. Outcomes after central grey matter injury in term perinatal hypoxic-ischemic encephalopathy. Early Hum Dev 86: 675–682, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med 336: 924–932, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Olivares L, Segovia A, Revuelta R. Tube feeding and lethal aspiration in neurological patients: a review of 720 autopsy cases. Stroke 5: 654–657, 1974. [DOI] [PubMed] [Google Scholar]
- 24.Omari TI, Benninga MA, Barnett CP, Haslam RR, Davidson GP, Dent J. Characterization of esophageal body and lower esophageal sphincter motor function in the very premature neonate. J Pediatr 135: 517–521, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Omari TI, Miki K, Fraser R, Davidson G, Haslam R, Goldsworthy W, Bakewell M, Kawahara H, Dent J. Esophageal body and lower esophageal sphincter function in healthy premature infants. Gastroenterology 109: 1757–1764, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Pena EM, Parks VN, Peng J, Fernandez SA, Di Lorenzo C, Shaker R, Jadcherla SR. Lower esophageal sphincter relaxation reflex kinetics: effects of peristaltic reflexes and maturation in human premature neonates. Am J Physiol Gastrointest Liver Physiol 299: G1386–G1395, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pensabene L, Miele E, DelGiudice E, Strisciuglio C, Staiano A. Mechanisms of gastroesophageal reflux in children with sequelae of birth asphyxia. Brain Dev 30: 563–571, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics 117: S28–S33, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Pickens DL, Schefft GL, Thach BT. Pharyngeal Fluid Clearance and Aspiration Preventive Mechanisms in Sleeping Infants. J Appl Physiol 66: 1164–1171, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Rangarathnam B, Kamarunas E, McCullough GH. Role of cerebellum in deglutition and deglutition disorders. Cerebellum 13: 767–776, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Saloojee H. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia: RHL commentary (last revised: 10 October 2007). In: The WHO Reproductive Health Library. Geneva, Switzerland: World Health Organization, 2007. [Google Scholar]
- 32.Sarnat H, Sarnat M. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 33: 696–705, 1976. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta JN. Electrophysiological recording from neurons controlling sensory and motor functions of the esophagus. Am J Med 111, Suppl 8A: 169S–173S, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med 111: 69–77, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, Sondheimer J, Staiano A, Thomson M, Veereman-Wauters G, Wenzl TG. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr 49: 498–547, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Volpe JJ. Neurology of the Newborn (5th ed). Philadelphia, PA: Elsevier, 2008. [Google Scholar]
- 37.Zanella S, Doi A, Garcia AJ, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci 34: 36–50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]