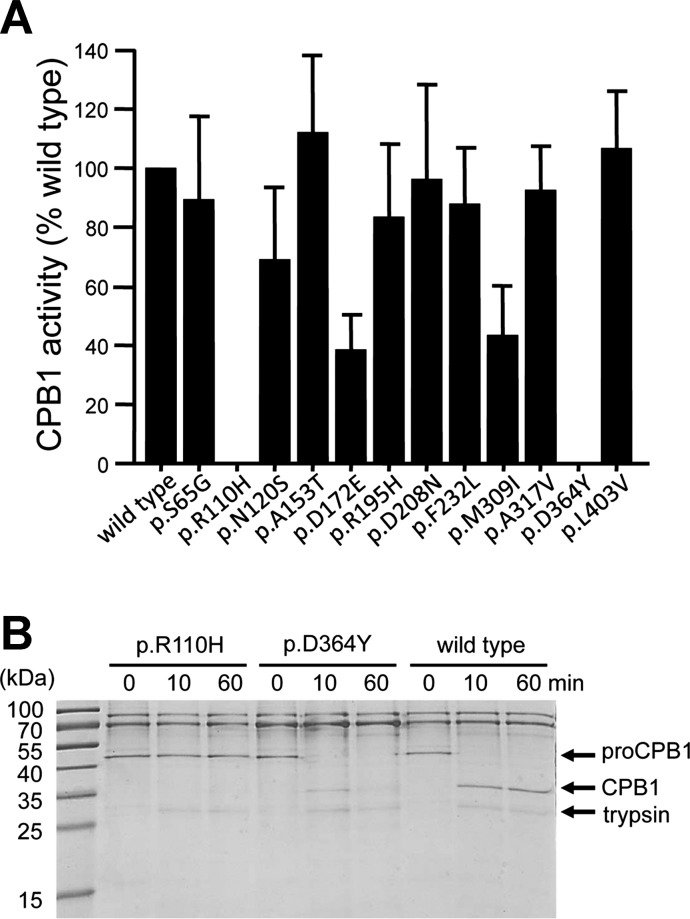
Fig. 3.
Activity and proteolytic stability of the CPB1 variants. A: activity of the CPB1 variants. CPB1 in the conditioned medium was activated with trypsin, and carboxypeptidase activity was measured as described in Methods. Activity was expressed as percent of the wild-type value. Averages from 4 transfections with SD are shown. B: proteolytic stability of CPB1 variants p.R110H and p.D364Y. Conditioned medium (110 μl/lane) was supplemented with 0.1 M Tris·HCl (pH 8.0) and 1 mM CaCl2 and treated with 50 nM human cationic trypsin for the indicated times (final concentrations). The reactions were stopped by precipitation with 10% trichloroacetic acid, and samples were analyzed by SDS-PAGE and Coomassie Blue staining. A representative gel from 2 experiments is shown.