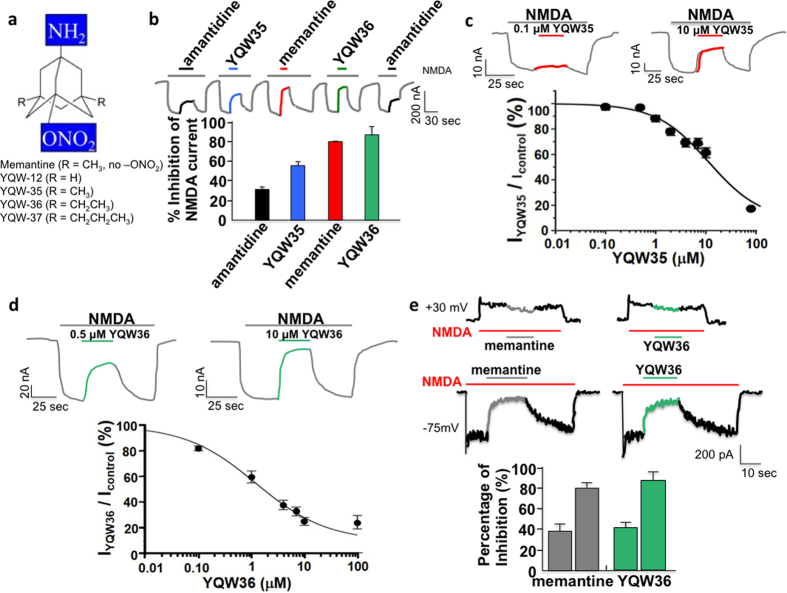
Figure 1. Channel block of NMDAR-mediated currents by various aminoadamantane drugs.
(a) Structure of aminoadamantane and aminoadamantane nitrates. 1-Aminoadamantane hydrochloride (memantine) has methyl side chains (‘R’ groups) and does not contain a nitro group off the back end of the molecule. With addition of the nitro group for redox functionality, the affinity of the aminoadamantane moiety for the NMDAR-associated ion channel is diminished. Lengthening the ‘R’ side chains, however, compensates for this. While memantine and YQW-035 have methyl side chains, YQW-012 has only protons, YQW-036 has ethyl groups, and YQW-037, propyl groups. Not only does lengthening the side chains increase binding affinity in the channel, but also increases the lipophilicity and thus penetrance of the blood-brain-barrier, while decreasing aqueous solubility. (b) Amantadine (10 μM) inhibited less NMDA current at steady state than equimolar memantine. Equimolar NitroMemantine YQW-035 manifests less channel block than memantine but more than amantadine. In contrast, NitroMemantine YQW-036 steady-state channel block of approximately the same degree as memantine. Two-electrode voltage clamp of oocytes expressing recombinant GluN1/GluN2A receptors at a holding potential (Vh) −70 mV. Values are mean + s.e.m. (c) Dose-response of NitroMemantine YQW-035 at Vh = −70 mV. Values are mean ± s.e.m. (d) Dose-response of NitroMemantine YQW-036 at Vh = −70 mV. Values are mean ± s.e.m. (e) In primary rat cortical neurons, whole-cell patch clamp recordings revealed that 5 μM memantine or NitroMemantine YQW-036 produced an approximately equivalent degree of blockade at both –75 mV and less blockade at +30 mV, indicating the voltage-dependence of the block by the memantine moiety. In the histogram, for each data point, n ≥ 5 recordings per drug tested; left bar in each pair for responses at −75 mV and right bar for +30 mV responses. Values are mean + s.e.m.