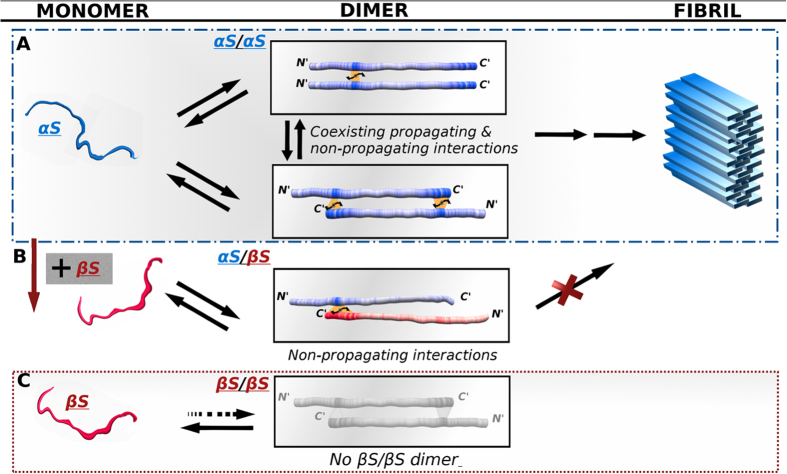
Figure 5. Schematic model of αS/αS and αS/βS transient interactions suggest a new molecular view of inhibition routes to αS aggregation.
(A) αS/αS homo-dimer interactions: head-to-head aggregation prone interactions at residues 36–44 co-exist with head-to-tail inhibitory interactions at residues 125–140. (B) αS/βS hetero-dimer interactions: head- to- tail inhibitory interactions span a broader range of C-terminal residues 115–134 with higher affinity. (C) No interactions are observed between monomer chains of βS. Protein chains are color-coded according to the interactivity of the regions as shown in Supplementary Fig. 4. The NMR data show that αS homo-dimers can sample a heterogeneous range of poulations including N-to-N and N- to C-terminal configurations while αS/βS hetero-dimers sample only N- to C- terminal configurations with higher affinity and specificity. In light of the fact that the fibril form of αS forms β-strands that assemble into in register parallel β-sheets this would suggest that the N-C terminal dimers would have to undergo conformational rearrangement to reach the final fibril form, thereby delaying or inhibiting the kinetics of fibril formation.