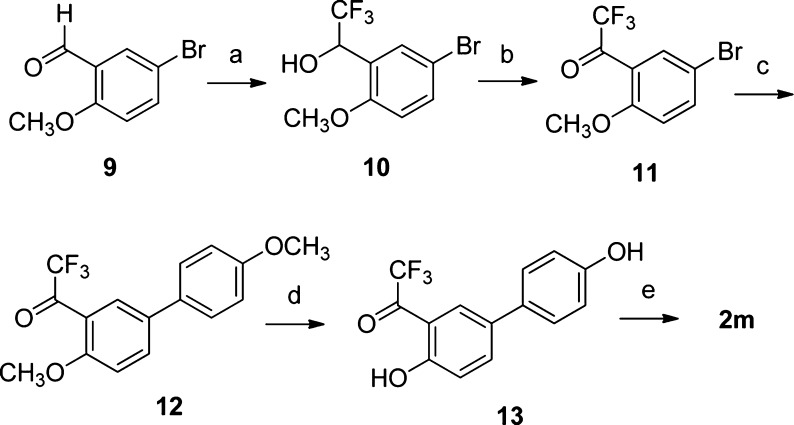
Scheme 2. Synthesis of Trifluoromethylketoxime 2m.
Reagents and conditions: (a) TMS–CF3, TBAF, THF, rt, 10 h; then HCl (4.4 M), rt, 1 h; (b) TEMPO, PhI(OAc)2 CH2Cl2, rt, 13 h; (c) 4-methoxyphenylboronic acid (1.2 equiv), Pd(OAc)2 (0.04 equiv), PPh3 (0.2 equiv), aqueous 2 M Na2CO3, 1:1 toluene/EtOH, 100 °C, 16 h; (d) BBr3, CH2Cl2, −78 to 0 °C, 1 h; (e) NH2OH·HCl, EtOH–H2O, 50 °C, 16 h.