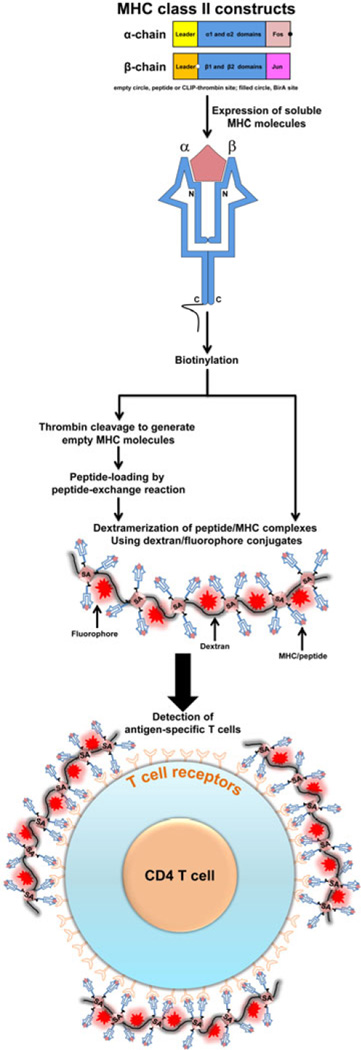
Figure 1. Derivation of MHC class II dextramers.
To produce soluble MHC class II monomers, two constructs, each representing MHC class II α and MHC class II β chains, are first created. Generally, we insert the nucleotide sequence for specific peptides of interest or thrombin-cleavage site-tagged/CLIP into the N-terminal end of the β chain construct. Likewise, Fos-and Jun-sequences are attached to the C-terminal ends of α and β constructs, respectively, to facilitate stability of α and β chains during protein expression, whereas the BirA site for biotinylation is introduced in the α-construct. The constructs are used to express soluble MHC molecules using Baculovirus in Sf9 insect cells. After biotinylation, the MHC monomers obtained from the constructs containing covalently tethered, specific peptide sequences are assembled with dextran molecules to obtain dextramers directly. In contrast, the biotinylated proteins generated from the CLIP-tethered constructs are first treated with thrombin to release the CLIP peptide, and the resulting empty MHC monomers are then used to load peptides of interest exogenously by peptide-exchange reaction. For dextramerization, the biotinylated MHC monomers generated from any of the above two approaches are mixed with dextran-SA-fluorochrome at molar ratios ranging from ~9:1 or 20:1, and the dextramers are then used to stain T cells to analyze their frequencies by flow cytometry.