Abstract
C3H/HeN female mice were vaccinated with native Chlamydia muridarum major outer membrane protein (MOMP), using Montanide+CpG or Alum+CpG as adjuvants. Negative control groups were immunized with ovalbumin (OVA) and the same adjuvants. As positive control, mice were inoculated intranasally with live Chlamydia. Mice were challenged in the ovarian bursa with 105 C. muridarum inclusion forming units. Six weeks after the genital challenge the animals were caged with male mice and monitored for pregnancy. Mice vaccinated with MOMP+Montanide+CpG developed high levels of C. muridarum-specific antibodies, with a high IgG2a/IgG1 ratio and neutralizing titres. Animals immunized using Alum+CpG had low antibody levels. Cellular immune responses were significantly higher in mice vaccinated with MOMP and Montanide+CpG, but not with Alum+CpG, when compared with negative controls. Following the genital challenge, only 20% (4/20) of mice vaccinated with MOMP+CpG+Montanide had positive vaginal cultures whereas 100% (9/9) of mice immunized with MOMP+CpG+Alum had positive cultures. Of the positive control animals inoculated with live Chlamydia only 15% (3/20) had positive vaginal cultures. In contrast, 100% (20/20) of mice immunized with OVA+CpG+Montanide, or minimal essential medium, had positive cultures. Following mating, 80% (16/20) of mice vaccinated with MOMP+CpG+Montanide, and 85% (17/20) of animals inoculated intranasally with live C. muridarum carried embryos in both uterine horns. No protection against infertility was observed in mice immunized with MOMP and CpG+Alum or OVA. In conclusion, this is the first time that a subunit vaccine has been shown to elicit a protective immune response in the highly susceptible C3H/HeN strain of mice against an upper genital challenge.
Keywords: adjuvants, Chlamydia muridarum, immunization, major outer membrane protein, mice
Introduction
Throughout the world millions of individuals are infected each year with Chlamydia trachomatis.1–3 The majority of patients infected in the genitourinary tract remain asymptomatic but others present with acute or chronic symptomatology including cervicitis, urethritis, abdominal pain, ectopic pregnancy and infertility.1,2,4 Ocular infections can also produce acute symptoms that can eventually result in trachoma.5,6 The reasons for this wide range of presentations and outcomes have been attributed to several pathogen- and host-related factors. For example, some isolates of C. trachomatis may be more virulent than others.7–9 In addition, host factors play a significant role in the outcome of the infection.10–13 For example, genetic factors can affect susceptibility to infection and the development of long-term sequelae.11–13 Specifically, Kinnunen et al.11 found the DQA1*0102 and DQB1*0602 genotypes significantly more frequently in patients with tubal factor infertility than in controls.
Chlamydia trachomatis isolates have been classified based on the cross-reactivity among serum samples and monoclonal antibodies generated by inoculating mice with the various serovars.14–16 Phylogenetic analysis of the nucleotide sequence of the major outer membrane protein (MOMP) supported the immunological classification of the C. trachomatis.17,18 This protein has four variable domains (VD), a trimeric β-barrel structure and porin function.17,19–21 The Chlamydia muridarum mouse pneumonitis (MoPn) isolate has been found to be able to infect mice of different genetic backgrounds.22–24 However, susceptibility to infection and development of long-term sequelae differ significantly from strain to strain of mice, mimicking the clinical presentations observed in humans.24–26
Screening for C. trachomatis and treating infected patients with antibiotics does not appear to have yielded the expected results. Several studies have shown that, following an initial decrease, there is a subsequent increase in the prevalence of C. trachomatis infections.27,28 The possibility that treating with antibiotics can result in a decline of natural immunity has been considered as an explanation for these findings.27,29,30 Hence, implementation of a vaccination programme has been proposed as a necessary strategy for decreasing the burden of chlamydial infections.31–38 The induction of an immune response by a vaccine is under genetic control.39,40 Therefore, before implementation in humans, it is necessary to test the efficacy of vaccines in animals with various genetic backgrounds.
Vaccines formulated with a native preparation of the MoPn MOMP can effectively protect BALB/c (H-2d) and C57BL/6 (H-2b) mice against chlamydial challenges.41–44 Here, we evaluated the efficacy of two vaccine formulations with native MOMP to protect C3H/HeN mice. This strain of mouse is exquisitely sensitive to chlamydial infections and highly prone to develop long-term sequelae, e.g. infertility. Therefore, C3H/HeN mice may be representative of humans susceptible to develop long-term sequelae.24 In addition, C3H/HeN mount a weak immune response to MOMP.45,46 Hence, engineering a vaccine to protect C3H/HeN mice may pose unique challenges that can provide valuable information for future implementation in humans. For these reasons and to improve the chances of uncovering an efficacious vaccine formulation we decided to compare two different types of combination adjuvants: one that includes adjuvants that favour a T helper type 1 (Th1) -biased immune response (CpG+Montanide), versus another adjuvant combination that favours a Th2-biased response (CpG+Alum). Here, for the first time, we have shown that a vaccine formulated with MOMP can protect C3H/HeN mice against genital challenge with Chlamydia.
Materials and methods
Stocks of Chlamydia
The C. muridarum [strain Nigg II; previously called Chlamydia trachomatis mouse pneumonitis (MoPn) biovar] was obtained from the American Type Culture Collection (ATCC; Manassas, VA).22,47 Chlamydia muridarum was grown in HeLa-229 cells with Eagle's minimal essential medium supplemented with 5% fetal calf serum.26 Elementary bodies (EB) were purified using Hypaque-76 (Nycomed Inc., Princeton, NJ) and stored at −70° in 0·2 m sucrose, 0·020 m sodium phosphate (pH 7·2) and 0·005 m glutamic acid.48
Purification of C. muridarum MOMP
Purification of native MOMP, directly from Chlamydia, has been described elsewhere.41,42 Briefly, C. muridarum was grown in McCoy monolayers, washed with PBS pH 7·4, centrifuged, and the pellet was treated with DNase. After centrifugation the pellet was resuspended in 0·2 m phosphate buffer pH 5·5, containing 0·1 m dithiothreitol, and 0·001 m each of EDTA and PMSF and extracted with CHAPS (Anatrace, Inc., Maumee, OH), and subsequently with Anzergent 3-14 (Z3-14; Anatrace, Inc.)49 The MOMP was purified using a hydroxyapatite column.48 The purified MOMP was refolded in the presence of reduced and oxidized glutathione. The preparation was concentrated and fixed with glutaraldehyde, and 2 m glycine was added to quench the reaction. The MOMP was concentrated using polyethylene glycol and dialysed against 0·02 m phosphate buffer pH 7·4, 0·15 m NaCl and 0·05% Z3-14 before immunization.
Animal immunization
Three-week-old female C3H/HeN (H-2k) mice were purchased from Charles River Laboratory (Wilmington, MA). Animals received a total of 10 μg of the MOMP, or ovalbumin (OVA; Sigma-Aldrich, St Louis, MO) per mouse per immunization.41,42 Animals were immunized intramuscularly (5 μg/mouse) and subcutaneously (5 μg/mouse) with MOMP. Adjuvants used were: 10 μg of CpG, [oligodeoxynucleotide-1826, (5′-TCCATGACGTTCCTGACGTT-3′); Coley Pharmaceutical Group, Kanata, ON], and Montanide ISA 720 (Seppic, Inc.; Fairfield, NJ) at a 3 : 7 volume/volume ratio of MOMP+CpG to Montanide, or 25 μl of Alum (Alhydrogel “85”; Superfos Biosector a/s; E.M. Sergeant Pulp & Chemical Co. Inc., Clifton, NJ).
Positive control C3H/HeN mice were immunized intranasally (i.n.) with 101 inclusion forming units (IFU)/mouse of C. muridarum in 40 μl of minimal essential medium (MEM).25,50 The number of IFU used for this inoculation was ∼10-fold lower than the 50% lethal dose (LD50) for C3H/HeN mice.50 A negative control group was immunized i.n. with 40 μl of MEM. A fertility control included animals that were not immunized, or challenged, but were mated in parallel with the other groups. All the experiments, except those involving Alum+CpG as adjuvants, were repeated. All mouse protocols were approved by the University of California, Irvine, Animal Care and Use Committee.
Antibody detection
Following immunization, blood samples were collected from the orbital plexus. Genital samples were collected by washing the vagina twice with 20 μl of PBS. Chlamydia muridarum-specific antibodies were measured in triplicate using an ELISA.26 Flat-bottom 96-well plates were coated with EB at a concentration of 10 μg/ml. A 1 : 1000 dilution of goat anti-mouse IgM, IgA, IgG (Cappel, Aurora, OH) and a 1 : 100 dilution of goat anti-mouse IgG1, IgG2a, IgG2b and IgG3 (Southern Biotechnology Associates, Birmingham, AL) was used to determine subclass or isotype-specific antibody.
The ability of serum to neutralize in vitro the infectivity of EB was determined as previously described.51 C. muridarum (104 IFU) were added to five-fold serial dilutions of the serum made with 5% guinea-pig sera in Ca2+,Mg2+-free PBS. After incubation at 37° for 45 min, the mixture was used to inoculate HeLa-229 cells by centrifugation. The cells were fixed with methanol at 30 hr after infection, stained with a pool of monoclonal antibodies prepared in our laboratory, and the number of IFU was counted. Neutralization was defined as ≥ 50% inhibition of the number of IFU using as a control the sera from the animals inoculated with OVA.
For immunoblotting, EB were resolved in 10% tricine–SDS–PAGE.52 A total of 20 µg of purified EB were loaded on a 7·5-cm-wide slab gel. Following transfer to nitrocellulose membranes, the non-specific sites were blocked with BLOTTO [Bovine Lacto Transfer Technique Optimizer: 5% (weight/volume) non-fat dried milk, 2 mm CaCl2, and 50 mm Tris–HCl, pH 8·0], and the serum samples were incubated overnight at 4°. Antibody binding was detected using horseradish peroxidase-conjugated goat anti-mouse antibody developed with 0·01% hydrogen peroxide and 4-chloro-1-naphthol. Monoclonal antibody MoPn-40 to MOMP was used as a control.
To detect antibodies elicited by vaccination to B-cell-specific linear epitopes, overlapping 25-mers corresponding to the mature MOMP amino acid sequence were chemically synthesized (SynBioSci Corp., Livermore, CA).53 Peptide 25 (p25) overlaps the N- and C-termini of MOMP. The peptides were adsorbed onto high binding affinity ELISA plates (10 μg/ml; 100 μl/well of a 96-well plate) and the antibody binding was determined in triplicates as described above using a 1 : 100 dilution of serum and a 1/10 000 dilution of anti-mouse IgG.54
Lymphocyte proliferation assay
A T-cell lymphoproliferative assay was performed using splenocytes as previously described.26 In brief, T-enriched cells were counted and 105 cells were aliquoted as 200 μl per well of a 96-well plate. UV-inactivated MoPn EB were added at a concentration of 10 EB to 1 antigen-presenting cells, which were prepared by irradiating splenocytes with 3300 rads. Negative control wells received medium alone and positive controls wells received concanavalin A at a concentration of 5 μg/ml. Cell proliferation was measured by addition of 1 μCi of [3H]thymidine per well. The mean count was obtained from triplicate cultures.
Measurement of cytokines
Levels of interferon-γ (IFN-γ) and interleukin-4 (IL-4) were determined using commercial kits in supernatants from splenic T cells stimulated as described above (BD Pharmingen, San Diego, CA).26
Genital challenge
Four weeks after the last immunization, mice were anaesthetized with xylazine and ketamine, a lateral abdominal incision was made, and 105 IFU of C. muridarum were inoculated into the left ovarian bursa.23,26 The right ovarian bursa was inoculated with mock-infected HeLa-229 cell extracts.
Vaginal cultures
To culture Chlamydia, vaginal swabs were collected at weekly intervals following the genital challenge.26 Swabs were vortexed in 200 μl of sugar phosphate glutamate and two samples from each specimen (100 and 10 μl) were inoculated into McCoy cells grown in 48-well plates. The plates were centrifuged at 1000 g for 1 hr at room temperature. Upon incubation at 37° for 30 hr they were fixed and stained with a pool of monoclonal antibodies to MoPn prepared in our laboratory.26
Fertility experiments
Six weeks after the genital challenge female mice were caged with male breeder mice.26 Starting at 10 days post-mating female mice were weighed every 3 days. Animals that gained weight were euthanized and the number of embryos was counted. Mice that did not gain weight were mated a second time with a different male mouse and the outcome of the mating was evaluated as indicated above.
Statistics
The two-tailed unpaired Student t-test, the Fisher's exact test and the Mann–Whitney U-test were employed to determine the significance of differences between the groups using the Statview software program on a Macintosh computer (Apple Co, Cupertino, CA).
Results
Antibody titres in sera and vaginal washes following vaccination
Following immunization serum samples were tested by an ELISA for the presence of C. muridarum-specific antibody. The antibody titres the day before the genital challenge, using EB as the antigen, are shown in Table1. High Chlamydia-specific antibody titres in serum were observed in the C3H/HeN animals vaccinated with MOMP+CpG+Montanide. The titre of total IgG in this group of mice was 51 200. In the same group of animals the titre of IgG2a was 51 200 and of IgG1 was 6400. The high ratio (8) of IgG2a/IgG1 is indicative of a Th1 response. In the group of animals immunized with MOMP+CpG+Alum the total IgG titre was 1600 and the IgG2a/IgG1 ratio was 2 (1600/800) consistent with a balanced Th1/Th2 response. Mice immunized i.n. with C. muridarum EB had an IgG serum titre of 12 800. This control group had high levels of IgG2a when compared with those of IgG1 (12 800/1600; ratio = 8), which was indicative of a strong Th1 response. Control groups immunized with OVA+CpG+Montanide, OVA+CpG+Alum, or MEM had no detectable antibody to Chlamydia.
Table 1.
Antibody titres the day before the genital challenge
| Antigen | Adjuvant | Chlamydia muridarum-specific ELISA antibody titre | Serum neutralizing titre | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Vaginal wash | ||||||||||
| IgM | IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgA | IgG | IgA | |||
| MOMP | CpG+Montanide | 100 | 51 200 | 6400 | 51 200 | 51 200 | 6400 | 1600 | 320 | 20 | 6250 |
| Ovalbumin | CpG+Montanide | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <10 | <10 | <50 |
| MOMP | CpG+Alum | <100 | 1600 | 800 | 1600 | 1600 | 800 | 200 | 20 | 10 | 250 |
| Ovalbumin | CpG+Alum | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <10 | <10 | <50 |
| Cm EB | <100 | 12 800 | 1600 | 12 800 | 12 800 | 3200 | 1600 | 40 | 160 | 1250 | |
| MEM | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <10 | <10 | <50 | |
EB, elementary body; MEM, minimal essential medium; MOMP, major outer membrane protein.
The neutralizing antibody titre in serum in the group of mice vaccinated with MOMP+CpG+Montanide was 6250. In contrast, in mice immunized with MOMP+CpG+Alum the neutralizing titre was 250 (Table1). In the control group inoculated i.n. with MoPn EB the neutralizing titre was 1250. Sera from mice immunized with OVA+CpG+Montanide, OVA+CpG+Alum, or MEM, served as the respective controls for the above three groups.
The titres of C. muridarum-specific IgA and IgG antibodies in vaginal washes in mice vaccinated with MOMP+CpG+Montanide were 20 and 320, respectively. In the group vaccinated with MOMP+CpG+Alum the titre of IgA in the vaginal washes was 10 and of IgG was 20. High levels of IgA, titre 160, were detected in the vaginal washes of the mice inoculated i.n. with live EB whereas the IgG titre in this group was 40. No Chlamydia-specific antibodies were detected in the vaginal washes of the control groups immunized with OVA or MEM.
Characterization of the antibody response with immunoblots and synthetic peptides
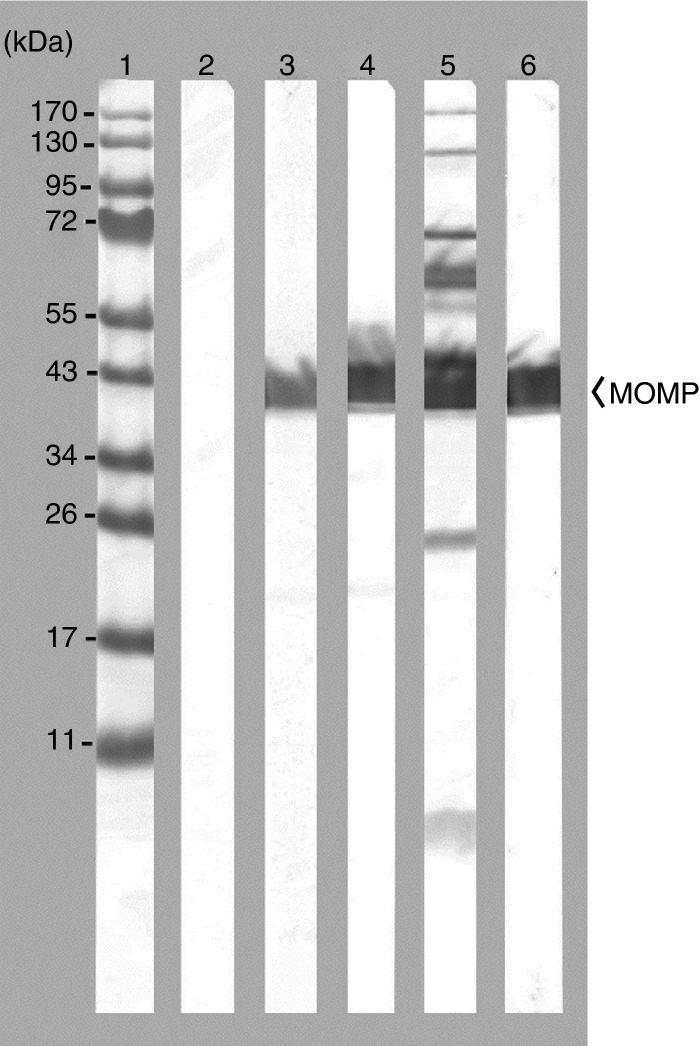
An immunoblot, using serum collected the day before the genital challenge, is shown in Fig.1. As expected, mice vaccinated with MOMP+CpG+Montanide, or MOMP+CpG+Alum, developed antibody only against MOMP. Animals immunized i.n. with EB had antibody predominantly to two bands of a high molecular weight (> 100 000), the 60 000 MW cysteine-rich protein, the 60 000 MW heat-shock protein, MOMP, a 23 000 MW protein and lipopolysaccharide. Control mice immunized with OVA, or MEM, had no antibody reactive with any of the chlamydial components.
Figure 1.
Immunoblot of Chlamydia muridarum elementary bodies (EB) probed with serum samples collected from immunized C3H/HeN mice. Lane 1, molecular weight standards; lane 2, serum sample diluted 1 : 100 collected before immunization. Serum samples collected the day before the genital challenge from mice immunized with the following: lane 3, major outer membrane protein (MOMP)+CpG+Montanide (serum dilution 1 : 40 000); lane 4, MOMP+CpG+Alum (serum dilution 1 : 10 000); lane 5, C. muridarumEB (serum dilution 1 : 100); lane 6, control monoclonal antibody MoPn-40 to MOMP (dilution 1 : 5).
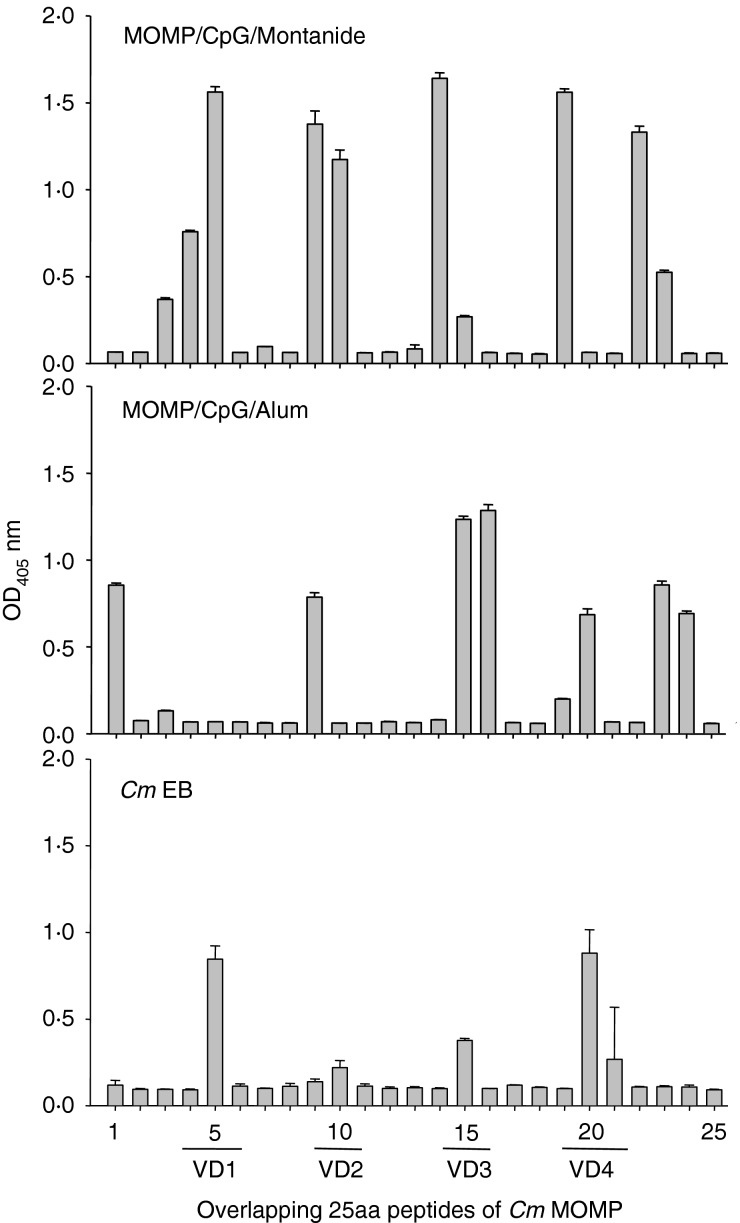
To determine the linear epitopes recognized by the antibody from mice immunized with MOMP, or EB, serum samples were tested against synthetic 25mer peptides of MOMP. As shown in Fig.2, IgG from mice vaccinated with MOMP+CpG+Montanide bound to the four VDs of MOMP. Lower levels of antibody were detected against VD2, VD3 and VD4 and no antibodies recognized VD1, in sera from mice vaccinated with MOMP+CpG+Alum. Sera from mice vaccinated with MOMP+CpG+Montanide or MOMP+CpG+Alum recognized peptides outside the VD whereas sera from animals immunized with EB only recognized epitopes in the VD.
Figure 2.
Mapping of antibodies to linear epitopes of major outer membrane protein (MOMP) using synthetic peptides. Overlapping 25mer peptides corresponding to the Chlamydia muridarumMOMP were incubated with serum samples from mice immunized with MOMP+CpG+Montanide (top), MOMP+CpG+Alum (middle) or live C. muridarum elementary bodies (EB) (bottom).
Determination of cell-mediated immune responses
T lymphocytes, stimulated with EB, from animals vaccinated with MOMP+CpG+Montanide showed an increase in their lymphoproliferative responses in comparison with the corresponding OVA control group [stimulation index (SI) 9·4 versus 1·9; P < 0·05; Table2]. No increase in the lymphoproliferative response was observed in the mice immunized with MOMP+CpG+Alum (SI 2·0 versus 2·4; P > 0·05). Control C3H/HeN mice inoculated i.n. with live C. muridarum showed a significant lymphoproliferative response to C. muridarum EB when compared with the MEM group (SI 80·0 versus 2·4; P < 0·05). The lymphoproliferative responses to concanavalin A and media, used as positive and negative controls, respectively, were equivalent among all the groups.
Table 2.
T-cell responses the day before the genital challenge1
| Antigen | Adjuvant | T-cell proliferation responses to: | In vitro cytokine production | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EB2 | Con A3 | Medium | IFN-γ (pg/ml) | IL-4 (pg/ml) | |||||||
| CPM x103 | SI4 | CPM x103 | SI4 | CPM x103 | SI4 | EB2 | Con A3 | EB2 | Con A3 | ||
| MOMP | CpG+Montanide | 1·3 ± 0·75 | 9·45,6 | 32·3 ± 9·2 | 223 | 0·2 ± 0·1 | 1 | 6682 ± 4025,6 | 77 892 ± 6850 | <0·8 | <0·8 |
| Ovalbumin | CpG+Montanide | 0·8 ± 0·7 | 1·9 | 29·0 ± 7·7 | 80 | 0·4 ± 0·2 | 1 | 4792 ± 612 | 80 668 ± 4650 | <0·8 | <0·8 |
| MOMP | CpG+Alum | 0·3 ± 0·1 | 2·0 | 33·9 ± 14·3 | 211 | 0·2 ± 0·1 | 1 | 2308 ± 724 | 76 805 ± 2987 | <0·8 | <0·8 |
| Ovalbumin | CpG+Alum | 0·5 ± 0·2 | 2·4 | 20·9 ± 5·7 | 99 | 0·2 ± 0·1 | 1 | 2272 ± 498 | 80 341 ± 1687 | <0·8 | <0·8 |
| Cm EB | 6·9 ± 2·85 | 80·05 | 36·6 ± 10·1 | 391 | 0·1 ± 0·02 | 1 | 72 149 ± 13 9105 | 71 820 ± 3964 | <0·8 | <0·8 | |
| MEM | 0·3 ± 0·1 | 2·4 | 43·3 ± 15·3 | 309 | 0·1 ± 0·1 | 1 | 1883 ± 791 | 70 052 ± 3863 | <0·8 | <0·8 | |
ConA, concanavalin A; EB, elementary body; IFN-γ, interferon-γ; IL-4, interleukin-4; MEM, minimal essential medium; MOMP, major outer membrane protein.
Results are means for triplicate cultures (±1 SD). Data correspond to one of the experiments representative of duplicate separate experiments.
UV-inactivated Chlamydia muridarum EB were added at a 10 : 1 ratio to the antigen-presenting cells.
Concanavalin A was added at a concentration of 5 µg/ml.
SI = stimulation index (CPM of EB stimulated/CPM of medium stimulated).
P < 0·05 by the Student's t-test, compared with the corresponding MEM-immunized control group.
P < 0·05 by the Student's t-test, compared with the corresponding ovalbumin-immunized control group.
The levels of IFN-γ from supernatants of splenocytes stimulated with C. muridarum EB in the mice vaccinated with MOMP+CpG+Montanide were elevated when compared with the respective OVA-immunized control groups (6682 versus 4792; P < 0·05; Table2). No increase in the levels of IFN-γ was observed in the group immunized with MOMP+CpG+Alum (2308 versus 2272; P > 0·05). The animals immunized i.n. with live Chlamydia had significantly higher levels of IFN-γ in the supernatants when compared with the control group immunized with MEM (72 149 versus 1883; P < 0·05). The levels of IL-4 were below the level of detection in all the groups (< 0·8 pg/ml).
Vaginal cultures
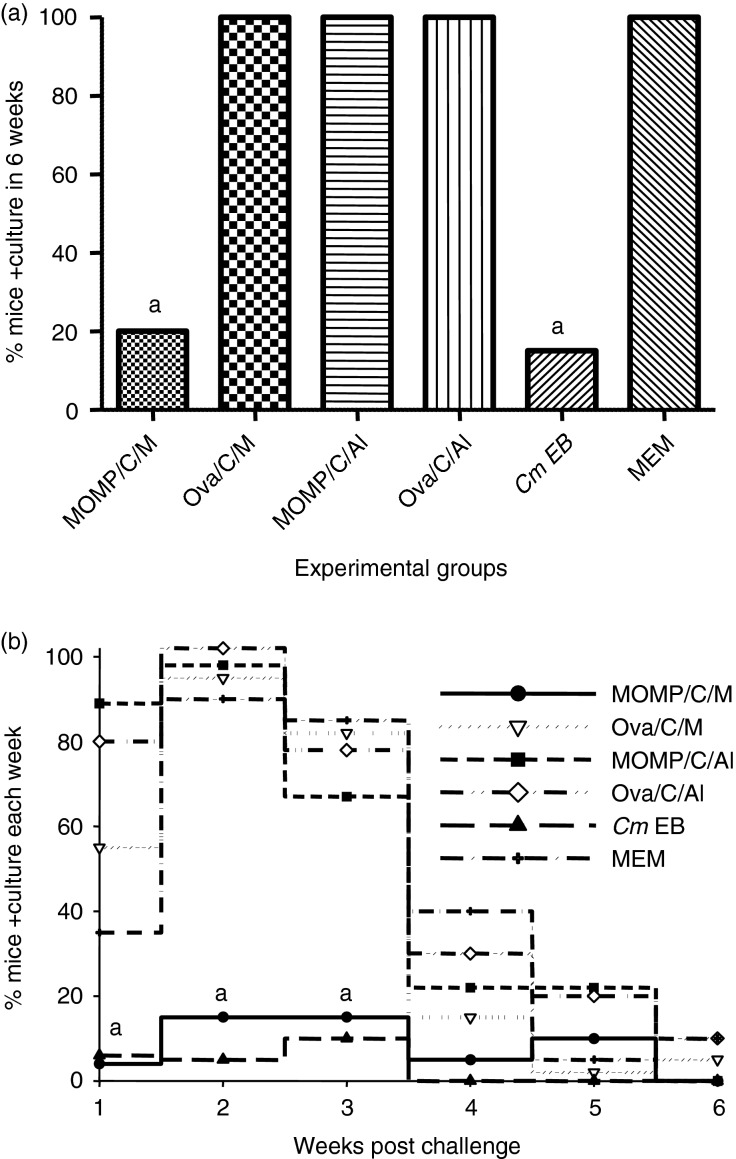
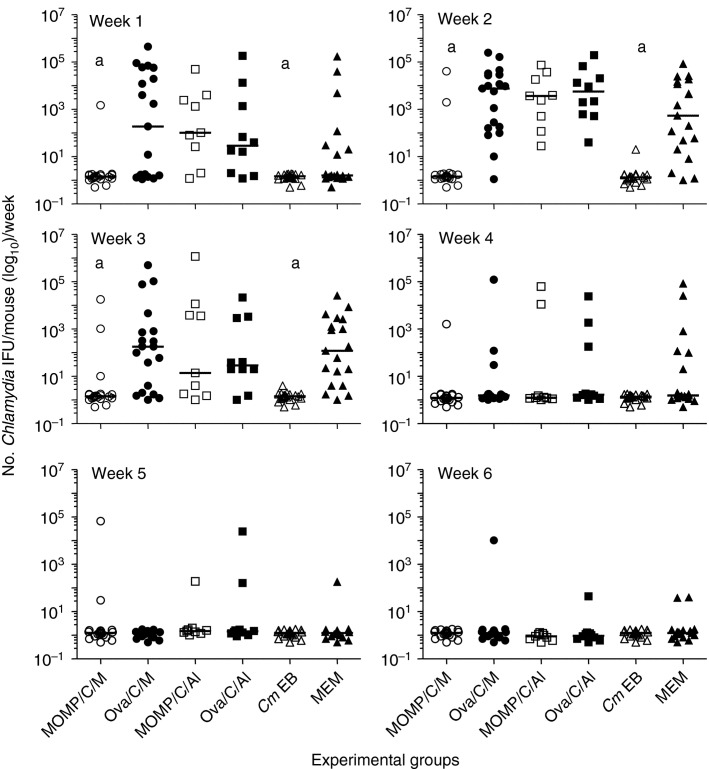
Four weeks after the last systemic immunization, mice were challenged in the left ovarian bursa with 105 IFU of C. muridarum and the course of the infection was assessed over a period of 6 weeks using vaginal cultures. As shown in Fig.3a, Chlamydia was recovered from the vaginal cultures in only 20% (4/20) of the mice vaccinated with MOMP+CpG+Montanide. Similarly, only 15% (3/20) of the mice immunized i.n. with live EB had positive vaginal cultures over the 6 weeks of observation. No statistically significant difference was found in the number of mice with positive vaginal cultures between these two groups (P > 0·05). All the animals (20/20) immunized with OVA+CpG+Montanide and the group of controls immunized with MEM had positive vaginal cultures. In addition, no statistically significant differences in the total or per week, number of IFU recovered, or in the length of vaginal shedding, were observed between the group vaccinated with MOMP+CpG+Montanide and the mice immunized i.n. with live EB (P > 0·05) (Figs3b and 4). The number of Chlamydia IFU recovered during the first 3 weeks after the challenge was significantly lower in the MOMP+CpG+Montanide-vaccinated animals than in the corresponding OVA+CpG+Montanide-immunized control group (P < 0·05). All animals vaccinated with MOMP+CpG+Alum, and the controls immunized with OVA+CpG+Alum, had positive vaginal cultures. Therefore, there was no significant statistical difference between the two groups (P > 0·05). Also, no differences in the number of IFU recovered, or in the length of the vaginal shedding, were noted between these two groups (P > 0·05).
Figure 3.
Percentage of mice with positive vaginal cultures. (a) Total percentage of mice that had positive vaginal cultures over the 6-week period. (b) Percentage of mice that had positive vaginal cultures each week of the 6 weeks of observation. Vaccinated mice were challenged with 105 inclusion forming units (IFU) of Chlamydia muridarum in the left ovarian bursa, vaginal cultures were collected weekly and percentage of mice with positive cultures was determined. aP < 0·05 by the Fisher's Exact test compared with the corresponding ovalbumin or minimal essential medium (MEM) -immunized control groups.
Figure 4.
Number of Chlamydia muridarum inclusion forming units (IFU) detected in the vaginal cultures. Vaccinated mice were challenged with 105 IFU of C. muridarum in the left ovarian bursa, vaginal cultures were collected weekly and the number of IFU present in each culture was determined. aP < 0·05 by the Mann–Whitney U-test compared with the corresponding ovalbumin or minimal essential medium (MEM) -immunized control groups.
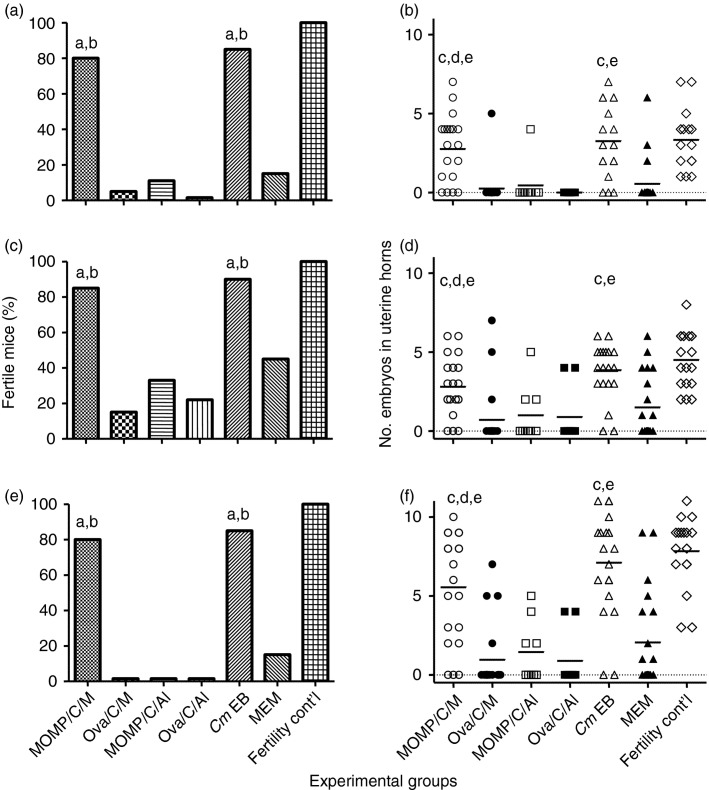
Fertility studies
Six weeks after the genital challenge, female mice were housed in the same cage with male mice and the pregnancy outcome was followed over a course of two mating cycles (Fig.5). Mice vaccinated with MOMP+CpG+Montanide, controls immunized i.n. with EB, and the fertility control group all had equivalent fertility rates in the left uterine horn: 80% (16/20), 85% (17/20) and 100% (18/18), respectively (P > 0·05). No protection against infertility was observed in mice vaccinated with MOMP+CpG+Alum when compared with the animals immunized with OVA+CpG+Alum (11% versus 0%; P > 0·05). The controls, immunized with OVA+CpG+Montanide, and the group inoculated with MEM had fertility rates of 5% (1/20) and 15% (3/20), respectively (P < 0·05).
Figure 5.
Seven weeks after the genital challenge female mice were caged with male breeder mice and their pregnancy status was followed in fertility studies. Mice that became pregnant were euthanized and the number of embryos was counted in each uterine horn. (a, c, e) Percentage fertile mice in the left, right and both uterine horns, respectively; (b, d, f) number of embryos in the left, right and both uterine horns, respectively. aP < 0·05 by the Fisher's Exact test when compared with the corresponding ovalbumin or minimal essential medium (MEM) -immunized negative control groups. bP > 0·05 by the Fisher's Exact test when compared with the fertility control group. cP < 0·05 by the Mann–Whitney U-test when compared with the corresponding ovalbumin- or MEM-immunized negative control groups. dP > 0·05 by the Mann–Whitney U-test when compared with the positive control elementary body (EB) -immunized group. eP > 0·05 by the Mann–Whitney U-test when compared with the fertility control group.
The number of embryos in the challenged left uterine horn, and the total number of embryos per mouse, were also equivalent for the MOMP+CpG+Montanide vaccinated group, the animals immunized i.n. with live Chlamydia and the fertility control mice. For example, in the group vaccinated with MOMP+CpG+Montanide, mice immunized i.n. with EB and in the fertility control group, the mean numbers of embryos in the left uterine horn were 2·8, 3·3 and 3·3, respectively (P > 0·05). Similarly, the means of the total number of embryos in the three groups 5·6, 7·1 and 7·8, respectively, were not significantly different (P > 0·05). In contrast, the total number of embryos in mice vaccinated with MOMP+CpG+Alum was not significantly different from the number of embryos in the animals immunized with OVA+CpG+Alum (1·4 and 0·9; P > 0·05). The groups immunized with OVA+CpG+Montanide, or with MEM, had significantly fewer embryos in both uterine horns, 1·0 and 2·1, respectively, than the group vaccinated i.n. with EB (P < 0·05).
Discussion
In this study we have shown that, a subunit vaccine formulated with the native MOMP, and adjuvants that favour a Th1 response, can induce a protective immune response in C3H/HeN (H-2k) female mice against a C. muridarum genital infection. Mice vaccinated with MOMP had a significant decrease in vaginal shedding that was similar to that observed in the control animals immunized with live EB. In addition, the fertility rates of MOMP vaccinated animals were equivalent to the fertility rates of the EB-immunized controls. These findings, in conjunction with previous results in BALB/c and C57BL/6 mice, provide support for the feasibility of engineering a subunit vaccine against C. trachomatis infections that can be effective in populations with different genetic backgrounds.41,43 Although others have reported protection against a vaginal challenge, to the best of our knowledge, only a subunit vaccine formulated with MOMP, and adjuvants that favour a Th1 response, have been shown to protect three strains of mice, with different genetic backgrounds, against an upper genital challenge with Chlamydia.41,43,44,55,56
The very high degree of polymorphism of the MHC molecules in the human population presents a difficult obstacle to overcome for the implementation of vaccines. Data from human studies and experimental models have shown that the ability of a vaccine to elicit a protective immune response is dependent, among other factors, on the genetic makeup of the individual.57–59 Addressing this issue, using subunit vaccines, is particularly important for pathogens such as C. trachomatis that are widespread throughout the world and affect individuals with very diverse genetic backgrounds. In subunit vaccines the number of protective epitopes is limited and, as a result, certain individuals may lack the MHC determinants needed to recognize the restricted number of epitopes. Furthermore, when using vaccines with live organisms, the in vivo replication of the attenuated pathogen increases the antigen load. In contrast, in the case of subunit vaccines, not delivered using replicating vectors, the amount of antigen is limited to the quantity inoculated at the time of immunization.
Vaccines for C. trachomatis infections were first tested to protect against trachoma. Immunization trials in humans and non-human primates provided encouraging results.2,6,32,60 Vaccines with the whole organism elicited protection although it was limited to a few years. Furthermore, the protection was, at least in part, serovar, or subgroup, specific. Individuals vaccinated with one of the serovars were protected against a subsequent exposure to the same, or related serovar, but not against one of the distantly related serovars.2,6,32,60 In addition, in certain individuals, exposure to C. trachomatis following vaccination resulted in a hypersensitivity reaction.60–63 It is considered that the hypersensitivity reaction occurs as a result of an immune response to one of the antigenic components present in Chlamydia. Although still under investigation, the 60 000 MW heat-shock protein, has been considered a candidate for this hypersensitivity reaction.64–70 As a result of the concerns with the hypersensitivity reaction current efforts have been focused on producing a subunit vaccine.
Using animal models, various components of both the humoral and the cell-mediated immune responses have been shown to play a role in protection against C. trachomatis infections.31,71–73 Passive immunization of mice with monoclonal IgA and IgG subclasses, to conformational epitopes of the C. muridarum MOMP, resulted in significant protection against vaginal and respiratory challenges.74,75 Work with C57BL/6 knockout mice indicates that CD4+ T cells are required for protection, whereas CD8+ T cells probably play a secondary role. Using C57BL/6 knockout mice, B cells and/or antibody have been shown to have a role in protection against a challenge with C. muridarum.43,71,72 Protection against MoPn seems to be mediated by Th1 cells as C57BL/6 animals deficient in IL-12, IFN-γ, or the IFN-γ receptor, cannot control an infection.31,71,72 However, for vaccine-induced protection, as in the case of re-infection, antibodies are also critical because they are as protective as CD4+ T cells.43,73,76,77 The very high level of protection against infection and disease shown in these experiments supports previous findings that linked antibodies to protection against upper genital pathology.41,75,78,79
Structural and immunological characterization of MOMP suggests that this protein mediated the serovar specificity of the protection observed in the trachoma vaccination trials.6,18,80,81 Taylor et al.82 immunized monkeys with MOMP and observed no protection against an ocular challenge. Other investigators using preparations of MOMP obtained similar disappointing results.83–85 Based on these results it was proposed that conformational epitopes present in MOMP might be required for protection.31,33,86,87 Support for this possibility was provided when, vaccines formulated with outer membrane complexes of Chlamydia, or with dendritic cells pulsed ex vivo with non-viable EB, were shown to induce significant protection against a genital challenge.41,42,84,86,88,89
In these experiments C3H/HeN mice immunized with native MOMP, and adjuvants that favour a Th2 response, were not protected against a genital challenge. In contrast, the immune response elicited in C3H/HeN mice by vaccination with the MoPn MOMP, and the Th1 adjuvant combination CpG+Montanide, was as protective as that resulting from intranasal inoculation with live Chlamydia. Therefore, as for BALB/c and C57BL/6 mice, a Th1 immune response appears to be necessary for protecting C3H/HeN mice against a genital challenge.44,72,73 Interestingly, the immune response of the C3H/HeN mice to this vaccination protocol is different from that observed in BALB/c and C57BL/6 mice. Overall, the C3H/HeN mice vaccinated with MOMP+CpG+Montanide had a strong humoral immune response and a weak cell-mediated immune response, whereas in BALB/c, or C57BL/6 mice the same immunization elicited more robust responses from both arms of the immune system.41,42,55,73 A weaker protection in C3H/HeNCrl (H2k), in comparison to BALB/c (H2d) and C57BL/6 (H2b) mice, was also recently reported by Yu et al.90 in animals immunized with combinations of polymorphic membrane proteins, recombinant MOMP and Th1 adjuvants. In these experiments they only obtained protection against vaginal shedding but not against hydrosalpinx formation.
To further dissect the differences between the responses of various strains of mice, we determined the linear epitopes of MOMP recognized by serum antibodies from the immunized mice. With this method we have previously shown that antibodies from BALB/c mice immunized with EB, or with MOMP, recognize all four VD of MOMP.26,55 In contrast, only antibodies from C3H/HeN vaccinated with MOMP recognized all VD whereas serum from mice immunized with EB had only a very weak response to the two dominant domains VD1 and VD4. Probably, animals immunized with EB, developed antibodies mainly to non-linear epitopes of MOMP, or to antigens other than MOMP, whereas mice immunized with MOMP had more robust humoral responses to linear epitopes of MOMP.
As a measure of the cellular immune response we used a lymphoproliferative assay using T-cells purified from the spleen. T cells, stimulated with EB, from EB- or MOMP-immunized BALB/c and C57BL/6 mice, mounted robust lymphoproliferative responses and high IFN-γ levels were measured in the supernatants.41,43 In contrast, in C3H/HeN mice weaker lymphoproliferative responses were observed in animals immunized with MOMP. However, a strong proliferative response, and high levels of IFN-γ in the supernatants, were demonstrated in mice immunized with live EB. Therefore, C3H/HeN mice do not produce very strong T-cell immune responses to MOMP. In this respect, Westbay et al.,46 immunized C3H/HeJ (H-2K), BALB/c (H-2d) and BALB.K (H-2k) with recombinant MOMP, using Alum as the adjuvant, and reported that, in contrast with the H-2d mice, the H-2k strains of mice were deficient in MOMP-specific helper T cells and had low Chlamydia-specific antibody levels. Based on these findings the authors questioned whether a vaccine formulated with MOMP could protect C3H/HeN (H-2K) mice. Similarly, Motin et al.45 showed that C3H/HeBkI (H-2k) mice had weaker lymphoproliferative responses to a peptide from a VD of MOMP than C57BL/6 animals. In spite of these observations, our results clearly show that C3H/HeN (H-2K) mice can be protected with a vaccine formulated with MOMP and Th1 adjuvants.
Until recently it was thought that protection induced by non-linear, or conformational, epitopes was dependent on antibody. In the last few years it has become evident that T cells can also recognize non-linear epitopes. For example, Musson et al.91 showed that the degree of antigen processing was dependent on the localization of the epitopes of the Yersinia pestis Caf1 protein. Epitopes located in the globular domains were presented by newly synthesized MHC class II, after low pH-dependent lysosomal processing, whereas epitopes from a flexible strand of the protein were presented by mature MHC class II, independent of low pH, and did not require proteolytic processing. These findings have now been confirmed by other investigators, who have demonstrated that the excision and splicing reactions of amino acids occurred in the proteasome.92 In the case of MOMP, conformational epitopes appear to play a significant role in the induction of protective immune responses.31,42,75 It is therefore possible that amino acid sequences from non-contiguous regions of the molecule may form new T-cell epitopes via post-translation transpeptidation in the host proteasome. As a result, both the protective humoral and the cell-mediated immune responses could, at least in part, be mediated by epitopes that are conformation dependent.
A limitation of this study is that we used the intrabursal challenge model developed by Swenson and Schachter.23 The main shortcoming of this model is that it does not parallel the natural route of infection. However, by directly challenging the site we are interested in protecting, it may provide a more stringent proof of the efficacy of the vaccine.73 The feasibility of determining the ability of Chlamydia to disseminate from one uterine horn to the other is another advantage of using the intrabursal versus the vaginal challenge. With the intravaginal model, to increase the susceptibility to infection and facilitate the development of infertility, the mice are treated with progesterone before they are challenged. Progesterone has strong immunomodulatory effects in mice and humans and induces a shift from Th1 to a Th2 response.93–95 A Th1 response is considered to be necessary for protection against a chlamydial infection. Hence, treating animals with progesterone before the challenge can mask the protective effects of a vaccine.93,94
In conclusion, vaccination of C3H/HeN mice with a native preparation of MOMP and adjuvants that favour a Th1 response elicits protective immune responses against a genital challenge as effective as that induced by immunization with live organisms. The results of the analyses of the immune responses in C3H/HeN, BALB/c and C57BL/6 mice indicate that different strains process purified MOMP differently from the one present in EB. A more detailed analysis is required to better understand the differences in the immune responses to vaccination between the strains, as this may help to improve the formulation of a vaccine for humans. Our results emphasize the need to test vaccination protocols in animals with different genetic backgrounds and stress the difficulties encountered in establishing immunological parameters that correlate with protection against chlamydial infections.
Acknowledgments
SP participated in the design of the experimental plan, performed the animal experiments and contributed to the writing of the manuscript. OT prepared stock reagents, helped to carry out the animal experiments and to perform the in vitro assays. LMM designed the experiments, monitored the execution of the in vivo and in vitro experiments and wrote the paper. This work was supported by Public Health Service grants AI-032248 and AI-092129 from the National Institute of Allergy and Infectious Diseases.
Glossary
- Cm
Chlamydia muridarum
- EB
elementary bodies
- IFN
interferon
- IL
interleukin
- i.n.
intranasal
- IFU
inclusion forming units
- MEM
minimum essential medium
- MOMP
major outer membrane protein
- MoPn
mouse pneumonitis
- OVA
ovalbumin
- SI
stimulatory index
- Th1
T helper type 1
- VD
variable domains
Disclosures
No conflicts of interests.
References
- Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291:2229–36. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- Schachter J, Dawson C. Human Chlamydial Infections. Littleton: PSG Publishing Co; 1978. [Google Scholar]
- Chlamydia screening among sexually active young female enrollees of health plans – United States, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009;58:362–5. [PubMed] [Google Scholar]
- Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–92. [PubMed] [Google Scholar]
- Grayston JT. Symposium on trachoma. Biology of the virus. Invest Ophthalmol. 1963;2:460–70. [PubMed] [Google Scholar]
- Taylor HR. Trachoma: A Blinding Scourge From the Bronze Age to the Twenty-First Century. 1st edn. Victoria, Australia: Haddington Press Pry Ltd; 2008. [Google Scholar]
- Xia M, Suchland RJ, Bumgarner RE, Peng T, Rockey DD, Stamm WE. Chlamydia trachomatis variant with nonfusing inclusions: growth dynamic and host-cell transcriptional response. J Infect Dis. 2005;192:1229–36. doi: 10.1086/444394. [DOI] [PubMed] [Google Scholar]
- Geisler WM, Suchland RJ, Whittington WL, Stamm WE. The relationship of serovar to clinical manifestations of urogenital Chlamydia trachomatis infection. Sex Transm Dis. 2003;30:160–5. doi: 10.1097/00007435-200302000-00013. [DOI] [PubMed] [Google Scholar]
- Carmichael JR, Tifrea D, Pal S, de la Maza LM. Differences in infectivity and induction of infertility: a comparative study of Chlamydia trachomatis strains in the murine model. Microbes Infect. 2013;15:219–29. doi: 10.1016/j.micinf.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Peterson EM, de la Maza LM. Susceptibility of mice to vaginal infection with Chlamydia trachomatis mouse pneumonitis is dependent on the age of the animal. Infect Immun. 2001;69:5203–6. doi: 10.1128/IAI.69.8.5203-5206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen AH, Surcel HM, Lehtinen M, Karhukorpi J, Tiitinen A, Halttunen M, et al. HLA DQ alleles and interleukin-10 polymorphism associated with Chlamydia trachomatis-related tubal factor infertility: a case–control study. Hum Reprod. 2002;17:2073–8. doi: 10.1093/humrep/17.8.2073. [DOI] [PubMed] [Google Scholar]
- Morre SA, Karimi O, Ouburg S. Chlamydia trachomatis: identification of susceptibility markers for ocular and sexually transmitted infection by immunogenetics. FEMS Immunol Med Microbiol. 2009;55:140–53. doi: 10.1111/j.1574-695X.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- Karimi O, Ouburg S, de Vries HJ, Pena AS, Pleijster J, Land JA, et al. TLR2 haplotypes in the susceptibility to and severity of Chlamydia trachomatis infections in Dutch women. Drugs Today (Barc) 2009;45(Suppl B):67–74. [PubMed] [Google Scholar]
- Wang S-P, Grayston J. Microimmunofluorescence serology of Chlamydia trachomatis. In: Peterson EM, de la Maza LM, editors. Medical Virology III. New York: Elsevier Science Pub; 1984. pp. 87–118. [Google Scholar]
- Wang SP, Grayston JT. Classification of trachoma virus strains by protection of mice from toxic death. J Immunol. 1963;90:849–56. [PubMed] [Google Scholar]
- Wang SP, Kuo CC, Barnes RC, Stephens RS, Grayston JT. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985;152:791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Sanchez-Pescador R, Wagar EA, Inouye C, Urdea MS. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–85. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WM, Peterson EM, de la Maza LM. Phylogenetic analysis of the outer-membrane-protein genes of Chlamydiae, and its implication for vaccine development. Mol Biol Evol. 1993;10:892–913. doi: 10.1093/oxfordjournals.molbev.a040048. [DOI] [PubMed] [Google Scholar]
- Yen TY, Pal S, de la Maza LM. Characterization of the disulfide bonds and free cysteine residues of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Biochemistry. 2005;44:6250–6. doi: 10.1021/bi047775v. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Maranon MJ, Bush RM, Peterson EM, Schirmer T, de la Maza LM. Prediction of the membrane-spanning β-strands of the major outer membrane protein of Chlamydia. Protein Sci. 2002;11:1854–61. doi: 10.1110/ps.3650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, et al. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol. 2007;189:6222–35. doi: 10.1128/JB.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg C. An unidentified virus which produces pneumonia and systemic infection in mice. Science. 1942;95:49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- Swenson CE, Schachter J. Infertility as a consequence of chlamydial infection of the upper genital tract in female mice. Sex Transm Dis. 1984;11:64–7. doi: 10.1097/00007435-198404000-00002. [DOI] [PubMed] [Google Scholar]
- de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–7. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamesipour A, Pal S, Peterson EM, de la Maza LM. Induction of infertility by the Chlamydia trachomatis mouse pneumonitis biovar in strains of mice that differ in their response to the 60 kDa heat shock protein. J Reprod Fertil. 1994;101:287–94. doi: 10.1530/jrf.0.1010287. [DOI] [PubMed] [Google Scholar]
- Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–62. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekart ML, Brunham RC. Epidemiology of chlamydial infection: are we losing ground? Sex Transm Infect. 2008;84:87–91. doi: 10.1136/sti.2007.027938. [DOI] [PubMed] [Google Scholar]
- Gotz H, Lindback J, Ripa T, Arneborn M, Ramsted K, Ekdahl K. Is the increase in notifications of Chlamydia trachomatis infections in Sweden the result of changes in prevalence, sampling frequency or diagnostic methods? Scand J Infect Dis. 2002;34:28–34. doi: 10.1080/00365540110077001. [DOI] [PubMed] [Google Scholar]
- Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–44. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- Patton DL, Teng A, Randall A, Liang X, Felgner PL, de la Maza LM. Whole genome identification of C. trachomatis immunodominant antigens after genital tract infections and effect of antibiotic treatment of pigtailed macaques. J Proteomics. 2014;108:99–109. doi: 10.1016/j.jprot.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–7. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- de la Maza LM, Peterson EM. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs. 2002;3:980–6. [PubMed] [Google Scholar]
- de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–27. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- Igietseme J, Eko F, He Q, Bandea C, Lubitz W, Garcia-Sastre A, et al. Delivery of Chlamydia vaccines. Expert Opin Drug Deliv. 2005;2:549–62. doi: 10.1517/17425247.2.3.549. [DOI] [PubMed] [Google Scholar]
- Rockey DD, Wang J, Lei L, Zhong G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines. 2009;8:1365–77. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- Mabey DC, Hu V, Bailey RL, Burton MJ, Holland MJ. Towards a safe and effective chlamydial vaccine: lessons from the eye. Vaccine. 2014;32:1572–8. doi: 10.1016/j.vaccine.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner LM, Wilson DP, Timms P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine. 2014;32:1563–71. doi: 10.1016/j.vaccine.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Yucesoy B, Johnson VJ, Fluharty K, Kashon ML, Slaven JE, Wilson NW, et al. Influence of cytokine gene variations on immunization to childhood vaccines. Vaccine. 2009;27:6991–7. doi: 10.1016/j.vaccine.2009.09.076. [DOI] [PubMed] [Google Scholar]
- Yucesoy B, Talzhanov Y, Johnson VJ, Wilson NW, Biagini RE, Wang W, et al. Genetic variants within the MHC region are associated with immune responsiveness to childhood vaccinations. Vaccine. 2013;31:5381–91. doi: 10.1016/j.vaccine.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;73:8153–60. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect Immun. 2002;70:4812–7. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun. 2010;78:4374–83. doi: 10.1128/IAI.00622-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Jensen KT, Follmann F, Agger EM, Theisen M, Andersen P. Liposome delivery of Chlamydia muridarum major outer membrane protein primes a Th1 response that protects against genital chlamydial infection in a mouse model. J Infect Dis. 2008;198:758–67. doi: 10.1086/590670. [DOI] [PubMed] [Google Scholar]
- Motin VL, de la Maza LM, Peterson EM. Immunization with a peptide corresponding to chlamydial heat shock protein 60 increases the humoral immune response in C3H mice to a peptide representing variable domain 4 of the major outer membrane protein of Chlamydia trachomatis. Clin Diagn Lab Immunol. 1999;6:356–63. doi: 10.1128/cdli.6.3.356-363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbay TD, Dascher CC, Hsia RC, Zauderer M, Bavoil PM. Deviation of immune response to Chlamydia psittaci outer membrane protein in lipopolysaccharide-hyporesponsive mice. Infect Immun. 1995;63:1391–3. doi: 10.1128/iai.63.4.1391-1393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett KD, Bush RM, Andersen AA. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999;49:415–40. doi: 10.1099/00207713-49-2-415. Pt 2: [DOI] [PubMed] [Google Scholar]
- Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–76. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MS, Gotschlich EC. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984;159:452–62. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Peterson EM, de la Maza LM. Induction of protective immunity against a Chlamydia trachomatis genital infection in three genetically distinct strains of mice. Immunology. 2003;110:368–75. doi: 10.1046/j.1365-2567.2003.01748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EM, Zhong GM, Carlson E, de la Maza LM. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988;56:885–91. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine–sodium dodecyl sulfate–polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Su H, Morrison RP, Watkins NG, Caldwell HD. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990;172:203–12. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Cheng X, Peterson EM, de la Maza LM. Mapping of a surface-exposed B-cell epitope to the variable sequent 3 of the major outer-membrane protein of Chlamydia trachomatis. J Gen Microbiol. 1993;139:1565–70. doi: 10.1099/00221287-139-7-1565. [DOI] [PubMed] [Google Scholar]
- Tifrea DF, Pal S, Popot JL, Cocco MJ, de la Maza LM. Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum. J Immunol. 2014;192:5201–13. doi: 10.4049/jimmunol.1303392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati M, Sambri V, Comanducci M, Di Leo K, Storni E, Giacani L, et al. DNA immunization with pgp3 gene of Chlamydia trachomatis inhibits the spread of chlamydial infection from the lower to the upper genital tract in C3H/HeN mice. Vaccine. 2003;21:1089–93. doi: 10.1016/s0264-410x(02)00631-x. [DOI] [PubMed] [Google Scholar]
- Lin HH, Liao HW, Lin SK, Wang LY. HLA and response to booster hepatitis B vaccination in anti-HBs-seronegative adolescents who had received primary infantile vaccination. Vaccine. 2008;26:3414–20. doi: 10.1016/j.vaccine.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Medina E, North RJ. Genetically susceptible mice remain proportionally more susceptible to tuberculosis after vaccination. Immunology. 1999;96:16–21. doi: 10.1046/j.1365-2567.1999.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubitz LF, Dial SM, Perrill R, Casement R, Galgiani JN. Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect Immun. 2008;76:5553–64. doi: 10.1128/IAI.00885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967;63(Suppl):1615–30. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- Collier LH, Blyth WA. Immunogenicity of experimental trachoma vaccines in baboons. I. Experimental methods, and preliminary tests with vaccines prepared in chick embryos and in HeLa cells. J Hyg (Lond) 1966;64:513–28. doi: 10.1017/s0022172400040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolridge RL, Grayston JT, Chang IH, Cheng KH, Yang CY, Neave C. Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma vaccine in Taiwan school children. Am J Ophthalmol. 1967;63(Suppl):1645–50. doi: 10.1016/0002-9394(67)94158-x. [DOI] [PubMed] [Google Scholar]
- Nichols RL, Bell SD, Jr, Haddad NA, Bobb AA. Studies on trachoma. VI. Microbiological observations in a field trial in Saudi Arabia of bivalent rachoma vaccine at three dosage levels. Am J Trop Med Hyg. 1969;18:723–30. [PubMed] [Google Scholar]
- Cohen CR, Koochesfahani KM, Meier AS, Shen C, Karunakaran K, Ondondo B, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-γ. J Infect Dis. 2005;192:591–9. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- Kinnunen A, Molander P, Morrison R, Lehtinen M, Karttunen R, Tiitinen A, et al. Chlamydial heat shock protein 60-specific T cells in inflamed salpingeal tissue. Fertil Steril. 2002;77:162–6. doi: 10.1016/s0015-0282(01)02922-3. [DOI] [PubMed] [Google Scholar]
- Kinnunen A, Paavonen J, Surcel HM. Heat shock protein 60 specific T-cell response in chlamydial infections. Scand J Immunol. 2001;54:76–81. doi: 10.1046/j.1365-3083.2001.00940.x. [DOI] [PubMed] [Google Scholar]
- Morrison RP, Lyng K, Caldwell HD. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989;169:663–75. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, Dascher C, Bowlin AK, Bavoil PM. Systemic immunization with Hsp60 alters the development of chlamydial ocular disease. Invest Ophthalmol Vis Sci. 1995;36:1344–51. [PubMed] [Google Scholar]
- Yi Y, Yang X, Brunham RC. Autoimmunity to heat shock protein 60 and antigen-specific production of interleukin-10. Infect Immun. 1997;65:1669–74. doi: 10.1128/iai.65.5.1669-1674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeling RW, Bailey RL, Conway DJ, Holland MJ, Campbell AE, Jallow O, et al. Antibody response to the 60-kDa chlamydial heat-shock protein is associated with scarring trachoma. J Infect Dis. 1998;177:256–9. doi: 10.1086/517367. [DOI] [PubMed] [Google Scholar]
- Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect Immun. 2000;68:6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun. 2011;79:986–96. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Bravo J, Peterson EM, de la Maza LM. Protection of wild-type and severe combined immunodeficiency mice against an intranasal challenge by passive immunization with monoclonal antibodies to the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Infect Immun. 2008;76:5581–7. doi: 10.1128/IAI.00574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–82. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175:7536–42. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–9. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–14. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AW, Follmann F, Erneholm K, Rosenkrands I, Andersen P. Protection against Chlamydia trachomatis infection and upper genital tract pathological changes by vaccine-promoted neutralizing antibodies directed to the VD4 of the major outer membrane protein. J Infect Dis. 2015;212:978–89. doi: 10.1093/infdis/jiv137. [DOI] [PubMed] [Google Scholar]
- Baehr W, Zhang YX, Joseph T, Su H, Nano FE, Everett KD, et al. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988;85:4000–4. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Wagar E, Schoolnik G. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988;167:817–31. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, Whittum-Hudson J, Schachter J, Caldwell HD, Prendergast RA. Oral immunization with chlamydial major outer membrane protein (MOMP) Invest Ophthalmol Vis Sci. 1988;29:1847–53. [PubMed] [Google Scholar]
- Su H, Caldwell HD. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992;175:227–35. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong-Ji Z, Yang X, Shen C, Lu H, Murdin A, Brunham RC. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect Immun. 2000;68:3074–8. doi: 10.1128/iai.68.6.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Barnhart KM, Wei Q, Abai AM, Peterson EM, de la Maza LM. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine. 1999;17:459–65. doi: 10.1016/s0264-410x(98)00219-9. [DOI] [PubMed] [Google Scholar]
- Batteiger BE, Rank RG, Bavoil PM, Soderberg LS. Partial protection against genital reinfection by immunization of guinea-pigs with isolated outer-membrane proteins of the chlamydial agent of guinea-pig inclusion conjunctivitis. J Gen Microbiol. 1993;139:2965–72. doi: 10.1099/00221287-139-12-2965. [DOI] [PubMed] [Google Scholar]
- Sun G, Pal S, Weiland J, Peterson EM, de la Maza LM. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine. 2009;27:5020–5. doi: 10.1016/j.vaccine.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–9. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–18. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Karunakaran KP, Jiang X, Brunham RC. Evaluation of a multisubunit recombinant polymorphic membrane protein and major outer membrane protein T cell vaccine against Chlamydia muridarum genital infection in three strains of mice. Vaccine. 2014;32:4672–80. doi: 10.1016/j.vaccine.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musson JA, Morton M, Walker N, Harper HM, McNeill HV, Williamson ED, et al. Sequential proteolytic processing of the capsular Caf1 antigen of Yersinia pestis for major histocompatibility complex class II-restricted presentation to T lymphocytes. J Biol Chem. 2006;281:26129–35. doi: 10.1074/jbc.M605482200. [DOI] [PubMed] [Google Scholar]
- Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–8. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- Abel K, Rourke T, Lu D, Bost K, McChesney MB, Miller CJ. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis. 2004;190:1697–705. doi: 10.1086/424600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–51. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushic C, Zhou F, Murdin AD, Wira CR. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun. 2000;68:4207–16. doi: 10.1128/iai.68.7.4207-4216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]