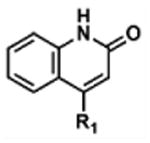
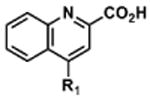
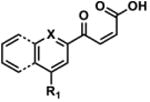
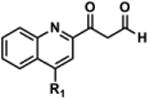
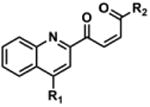
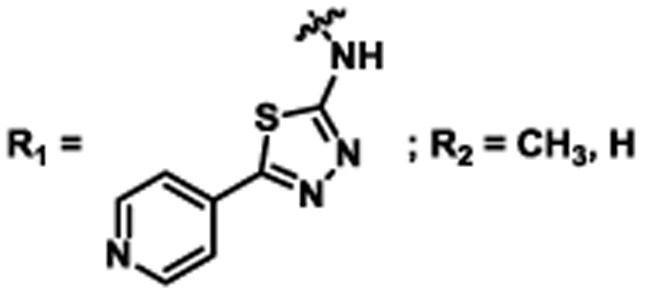
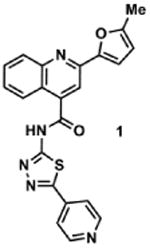
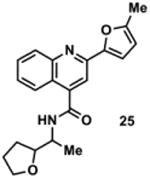
Table 2. Decomposition of Furan-Functionalized Quinolines in DMSO Solution.
| Decomposition Products Observedc,d | ||||||||
|---|---|---|---|---|---|---|---|---|
| HTS Library Scaffolda | PubChem CID | Exact Mass | % Parent Remaining ± SD (38 days)b |
|
|
|
|
|
|
||||||||
|
1257050 | 413.1 | 6 ± 1 | + 350.1 (350.1) | + 378.1 (378.1) | + 432.1 (432.1) | + 404.1 (404.1) | + 430.1 (430.1) |
|
2965431 | 350.2 | 55 ± 4 | + 287.1 (287.1) | + 315.1 (315.1) | - | + 341.4 (341.1) | + 367.2 (367.2) |
|
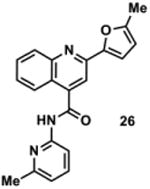
845057 | 343.1 | 73 ± 8 | + 280.1 (280.1) | + 308.1 (308.1) | + 362.1 (362.1) | + 334.2 (334.1) | + 360.2 (360.1) |
|
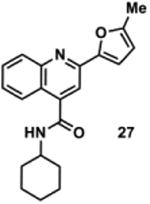
898877 | 334.2 | 54 ± 19 | + 271.1 (271.1) | + 299.1 (299.1) | + 353.2 (353.1) | + 325.2 (325.1) | + 351.2 (351.2) |
|
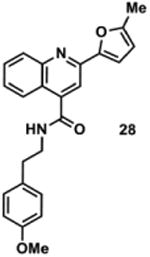
1290240 | 386.2 | 84 ± 32 | + 323.1 (323.1) | + 351.2 (351.1) | + 405.2 (405.1) | + 377.2 (377.1) | + 403.2 (403.2) |
|
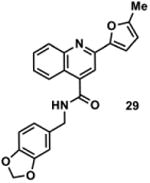
1001397 | 386.1 | 56 ± 28 | + 323.1 (323.1) | + 351.2 (351.1) | + 405.1 (405.1) | + 377.1 (377.1) | + 403.1 (403.1) |
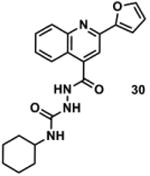
|
1295580 | 378.2 | 64 ± 19 | + 329.1 (329.1) | + 357.1 (357.1) | +e 411.1 (411.1) | + 383.1 (383.1) | - |
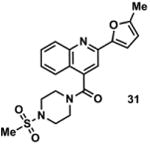
|
2220790 | 399.1 | 26 ± 4 | + 336.1 (336.1) | + 364.1 (364.1) | + 418.1 (418.1) | + 390.1 (390.1) | + 416.1 (416.1) |
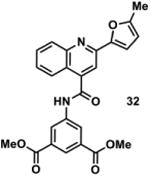
|
1000751 | 444.1 | 19 ± 10 | + 381.1 (381.1) | + 409.1 (409.1) | + 463.1 (463.1) | - | + 461.1 (461.1) |
Available in either the NIH MLPCN collection or the University of Minnesota small molecule collection.
10 mM solutions in DMSO were shaken gently at 25 °C open to the atmosphere. Percent remaining is the mean of three independent experiments.
DMSO stock solutions were analyzed by LC-MS on day 38. Chromatograms and spectra can be found in the SI (Sections XXXII – XXXIX). Decomposition products positively identified are denoted by +, while products not detected are denoted by -. For observed decomposition products, the masses are presented as found mass and calculated mass in parentheses. Masses were calculated for [M+H]+.
The aldehyde decomposition product could also be the enol tautomer.
For probes with a non-methylated furan, the carboxylic acid and [M+O2+H]+ intermediates (highlighted in yellow in Figure 4) have the same MW. The enone is represented in this table as identified; however, the observed m/z may correspond to an [M+O2+H]+ intermediate.
