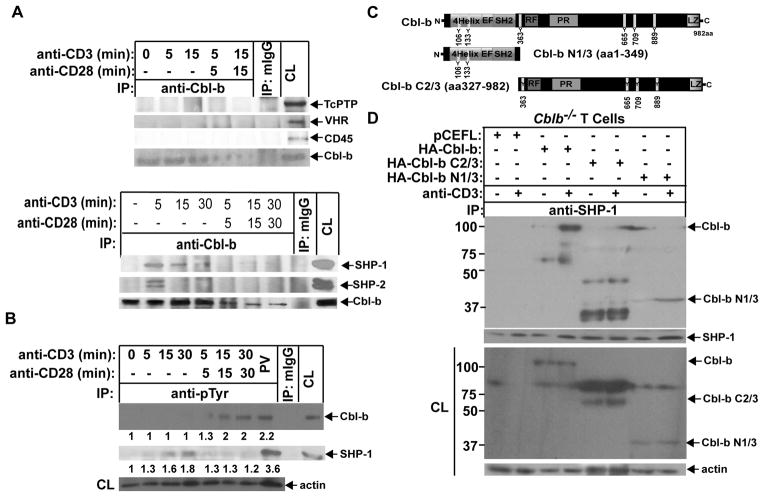
FIGURE 2.
SHP-1 binds to Cbl-b upon TCR stimulation via its TKB domain. (A) B6 T cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 for times indicated, and lysed in 0.5% NP-40 lysis buffer. The cell lysates were immunoprecipitated with anti-Cbl-b and blotted with anti-TcPTP, anti-CD45, anti-VHR, anti-SHP-1, and anti-SHP-2. (B) B6 T cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 for times indicated, and lysed in RIPA buffer. The cell lysates were immunoprecipitated with anti-pTyr, and blotted with anti-anti-Cbl-b and anti-SHP-1, respectively. The equal loading was confirmed by immunolblotting the cell lysates with anti-actin. B6 T cells treated with PV for 15 min were used as positive control. (C) Schematic design of Cbl-b mutants. (D) Cblb−/− T cells were retrovirally transfected with HA-FL Cbl-b, Cbl-b N1/3, and Cbl-b C2/3. The transfected cells were stimulated with anti-CD3, and lysed. The cell lysates were immunoprecipitated with anti-SHP-1 and blotted with anti-HA. The fold changes of protein bands are indicated in arbitrary densitometric units. Data are representative of at least three independent experiments.