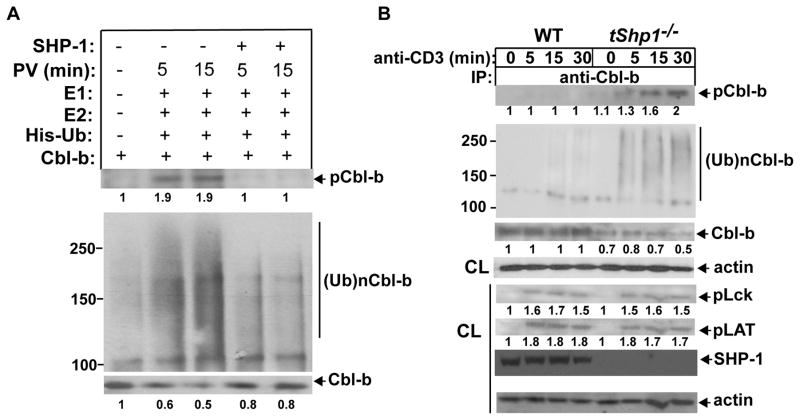
FIGURE 3.
SHP-1 prevents Cbl-b tyrosine phosphorylation and ubiquitination. (A) B6 T cells pretreated with pervanadate or left untreated were lysed in RIPA buffer, and immunoprecipitated with anti-Cbl-b. Cbl-b immunoprecipitates were incubated with recombinant, active SHP-1 for 30 min, and extensively washed with RIPA buffer to remove SHP-1 which may potentially bind to Cbl-b. SHP-1-treated or untreated Cbl-b immunoprecipitates were incubated with E1, Ubc5, and His-tagged ubiquitin, and blotted with anti-pTyr and anti-His. (B) Naïve CD4+ T cells from Cd4 Cre and tShp1−/− mice were stimulated with anti-CD3, and lysed in 1% RIPA buffer. The cell lysates were immunoprecipitated with anti-Cbl-b, and blotted with anti-pTyr, anti-ubiquitin, and anti-Cbl-b, respectively (upper panel). The lysates from Cd4 Cre and tShp1−/− T cells were blotted with anti-phospho-Lck, anti-phospho-LAT, anti-SHP-1, and anti-actin (lower panel). The fold changes of protein bands are indicated in arbitrary densitometric units. Data are representative of three independent experiments.