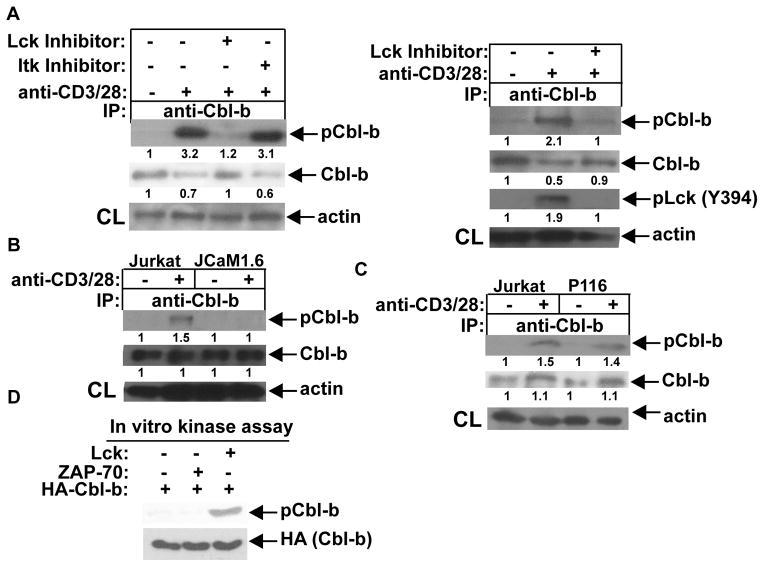
FIGURE 4.
Lck is the PTK that phosphorylates Cbl-b. (A) B6 T cells were pretreated with specific inhibitors of Lck or ITK (left panel), or Lck inhibitor (right panel), for 30 min, stimulated with anti-CD3 and anti-CD28 for 15 min, and lysed. The cell lysates were immunoprecipitated with anti-Cbl-b, and blotted with anti-pTyr (left panel). The cell lysates were blotted with anti-pLck (Y394) and anti-Cbl-b, and reprobed with anti-actin (right panel). (B and C) Parental Jurkat cells, JCaM1.6 cells, or P116 cells were stimulated with anti-CD3 and anti-CD28, and lysed. Cbl-b tyrosine phosphorylation was determined. (D) Recombinant, active Lck and ZAP-70 were incubated with HA-Cbl-b in the kinase buffer in the presence of ATP. After stopping the reaction, the mixture was blotted with anti-pTyr. The fold changes of protein bands are indicated in arbitrary densitometric units. Data are representative of two independent experiments.