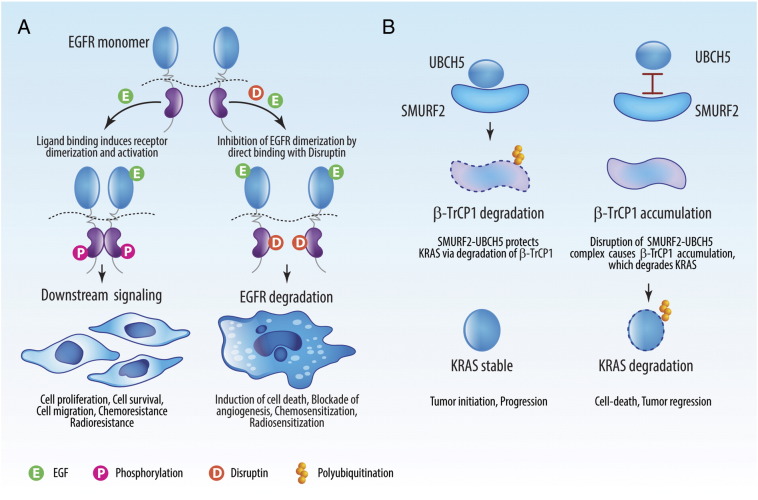
Figure 1.
Disruption of protein-protein interactions to induce oncoproteins degradation. (A) Disruptin (D), a peptide containing an eight–amino acid stretch of human EGFR, is capable of disrupting the protein:protein interactions between EGFR:HSP90 and EGFR homodimers. These interactions are critical for maintaining EGFR stability. In the presence of high levels of EGF, as seen in the tumor microenvironment, treatment with Disruptin causes rapid degradation of EGFR. In preclinical models, treatment with Disruptin induces regression of EGFR-dependent tumors including those resistant to TKIs. (B) Interaction between the SMURF2:UBCH5 protein complex and β-TrCP1 plays a critical role in the maintenance of KRAS protein stability. The SMURF2:UBCH5 complex polyubiquitinates and degrades β-TrCP1, which indirectly protects KRAS from degradation. We found that the disruption of the SMURF2:UBCH5 complex can promote β-TrCP1 accumulation, leading to rapid KRAS degradation.