Figure 8.
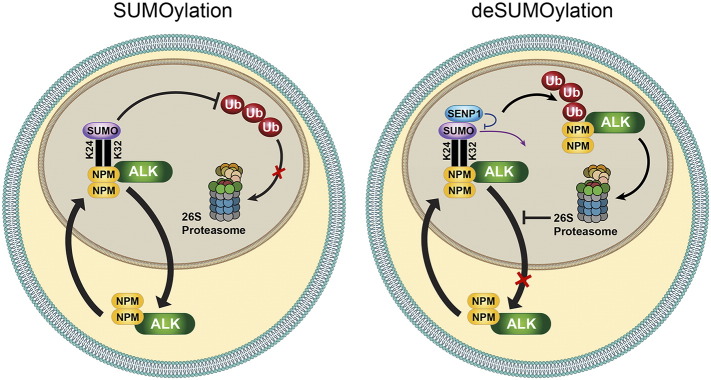
Proposed model for NPM-ALK SUMOylation. NPM-ALK forms heterodimers with wild-type NPM. These NPM/NPM-ALK heterodimers possess ability to shuttle between the nucleus and cytoplasm. The association between NPM/NPM-ALK, through its Lys24 and Lys32 residues, with the SUMO proteins occurs predominantly in the nucleus, which is the cellular compartment where SUMO proteins appear to be predominantly expressed in NPM-ALK+ T-cell lymphoma cells. In turn, conjugation with the SUMO modifiers confers stability on NPM-ALK oncogenic protein by preventing its ubiquitination and degradation. Therefore, NPM/NPM-ALK is capable of shuttling back to the cytoplasm. To this end, SUMOylation-mediated stability leads to accumulation and abundant expression of NPM-ALK in the nucleus and cytoplasm. At the other hand, SENP1-mediated de-SUMOylation decreases significantly the expression and accumulation of NPM/NPM-ALK in the nucleus and cytoplasm through the increase in NPM-ALK breakdown that occurs through the switch from the protective effects of SUMOylation to the ubiquitination-induced degradation.
