Supplemental Figure 2.
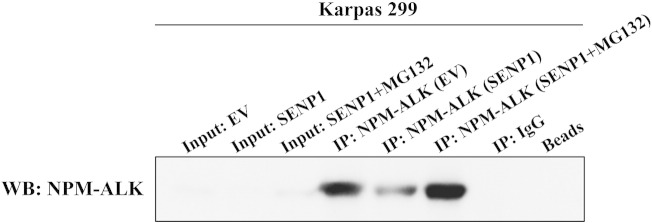
SENP1-induced decrease in levels of NPM-ALK is reversed by MG132. Treatment of Karpas 299 cells with SENP1 induced de-SUMOylation of NPM-ALK protein (Figure 5), which was associated with a remarkable decrease in the basal levels of NPM-ALK protein most likely through an increase in its association with ubiquitin (Figure 6). In this experiment, we show that SENP1-mediated downregulation of NPM-ALK protein levels was reversed when the proteasome inhibitor MG132 was simultaneously used to treat the cells with SENP1. These data further suggest that de-SUMOylation directs NPM-ALK protein to ubiquitination and proteasomal degradation.
