Abstract
AIM: To investigate the global gene expression of cancer related genes in hepatoma cell line HLE using Atlas Human Cancer Array membranes with 588 well-characterized human genes related with cancer and tumor biology.
METHODS: Hybridization of cDNA blotting membrane was performed with 32P-labeled cDNA probes synthesized from RNA isolated from Human hepatoma cell line HLE and non-cirrhotic normal liver which was liver transplantation donor. AtlasImage, a software specific to array, was used to analyze the result. The expression pattern of some genes identified by Atlas arrays hybridization was confirmed by reverse transcription polymerase chain reaction (RT-PCR) in 24 pairs of specimens and Northern blot of 4 pairs of specimens.
RESULTS: The differential expression of cell cycle/growth regulator in hepatocellular carcinoma (HCC) showed a stronger tendency toward cell proliferation with more than 1.5-fold up-regulation of Cyclin C, ERK5, ERK6, E2F-3, TFDP-2 and CK4. The anti-apoptotic factors such as Akt-1 were up-regulated, whereas the promotive genes of apoptosis such as ABL2 were down-regulated. Among oncogene/tumors suppressors, SKY was down-regulated. Some genes such as Integrin beta 8, Integrin beta 7, DNA-PK, CSPCP, byglycan, Tenacin and DNA Topo were up-regulated. A number of genes, including LAR, MEK1, eps15, TDGF1, ARHGDIA were down-regulated. In general, expression of the cancer progression genes was up-regulated, while expression of anti-cancer progression genes was down-regulated. These differentially expressed genes tested with RT-PCR were in consistent with cDNA array findings.
CONCLUSION: Investigation of these genes in HCC is helpful in disclosing molecular mechanism of pathogenesis and progression of HCC. For the first time few genes were discovered in HCC. Further study is required for the precise relationship between the altered genes and their correlation with the pathogenesis of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and ranks the eighth in incidence of human cancer in Asia, Africa and South Europe, and causes an estimated 1 million deaths annually. The molecular mechanism underling HCC is currently unknown[1-15]. Tumor development and progression involves a cascade of genetic alterations. Techniques frequently used in study of gene expression, such as RT-PCR, differential display PCR and Northern blot analysis, have their limitations including requirement of large amounts of RNA, time-consuming, and limited number of genes being tested simultaneously. Hence, analysis of expression profiles of a large number of genes in hepatoma cell line is an essential step toward clarifying the detailed mechanisms of hepatocarcinogenesis and discovering target molecules for the development of novel therapeutic drugs.
DNA microarray enables investigators to study the gene expression profile and gene activation in thousands of genes and sequences[16-25]. In this study, we used cDNA expression microarray containing 588 genes related to carcinoma to analyze genes that are differentially expressed in human Hepatoma cell line HLE.
MATERIALS AND METHODS
Tissues and specimens
Four normal liver tissues without cirrhosis and 24 pairs of primary HCC and corresponding noncancerous liver tissues without cirrhosis were obtained with informed consent from patients who underwent liver transplantation and hepatectomy at the First Clinical College of Harbin Medical University. Histopathological identification was confirmed by the same pathologist. These specimens were immediately frozen in liquid nitrogen once obtained.
Cell culture
The HCC cell line HLE, epithelial-like cells, established from HCC patient in 1975 by Dr Dor was used in this study. HLE was cultured in RPMI1640 (Sigma, Saint Louis, USA) media containing 10% fetal bovine serum, 1% penicillin and streptomycin in a 37 °C incubator. Cells were harvested at 70%-80% confluence.
RNA isolation and purification
Total RNA of normal liver tissues was obtained by extracting frozen tissues in Trizol (Life Technologies Inc., Gaitherbur, MD) according to the manufacturer’s instructions. Normal liver were made in spices and homogenized in Trizol solution (1 ml/100 mg). Trizol was added into the bottles cultured with HLE, after washed with cold PBS. The concentration of RNA was assessed by absorbency at 260 nm using a Nucleic Acid and Protein Analyzer (BECKMAN 470, USA).
cDNA microarray membrane
Atlas human cancer cDNA expression array (7742-1) was purchased from Clonech Laboratories Inc (Palo Alto, USA). The membrane contained 10 ng of each gene-specific cDNA from 588 known genes and 9 housekeeping genes. The cancer-related genes analyzed in this study were divided into six different groups according to its function.
cDNA synthesis, labeling and purification
Total RNA was reverse-transcribed into cDNA and labeled with α-32P dCTP using SuperscriptTM Preamplification system for First Strand cDNA Synthesis Kit (Life Technologies, Gaithersburg, MD) following the manufacture’s instructions. The labeled first strand cDNA probes were purified by Spin 200 column (Clontech, Palo Alto, CA) to remove the unincorporated nucleotides.
Membrane hybridization and exposure
Different probes were added to tubes containing Atlas human cancer cDNA expression array, which were pre-hybridized at 68 °C for 2 hr, and hybridization was performed at 68 °C for 18 hr in rolling bottles. The membranes were washed strictly and exposed to X-ray films (Fuji Films, Tokyo, Japan) at -70 °C for 1-3 d.
Image and analysis
The images were scanned with Fluor-S MultiImager (Bio-Rad, Hercules, CA) and analyzed with AtlasImage analysis software Version 1.01a (Clontech, Palo Alto, CA). Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected for normalization because its expression was constant in cancer array hybridization system. The normalized intensity of each spot representing a unique gene expression level was acquired. Genes were considered to be up-regulated when the intensity ratio was ≥ 1.5 or the difference was ≥ 10000 between the expressions of HLE and normal liver tissues.
Semi-quantitative RT-PCR
To confirm the cDNA array results, semi-quantitative RT-PCR of 24 pairs of HCC tissues and normal liver tissues was performed for two genes (TFDP2, E2F3) displaying expression alterations. Twenty-five ml reaction mixture was performed under the following conditions: denaturation at 95 °C (3 min); 24 cycles of 94 °C (30 s), 60 °C (30 s) and 72 °C (45 s); then 72 °C extension (3 min). GAPDH were used as an internal reference in each PCR reaction. The 5 ml RT-PCR product was analyzed by electrophoresis on a 1.5% agarose gel.
Primer were as follows: GAPDH, forward primer 5’-ACCACAGTCCATGCCATCAC-3’ and reverse primer 5’-TCCACCACCCTGTTGCTGTA-3’; TFDP2, forward primer 5’-GGAGTCAGGCAAATGCTCTC-3’ and reverse primer 5’-GCTAAGGCCACTTCTGCATC-3’; E2F3, forward primer 5’-TTATGACTGCGTGAGCCTTAG-3’ and reverse primer 5’-AGAGCCACAACAAAGAACAGA-3’.
Northern blot
RNAs of HCC and normal liver tissues were electrophoresed in a 1.5% agarose gel containing 2.2 M formaldehyde, and then transferred onto a nylon membrane (Zeta-Probe, Bio-Rad, USA) by capillary action. RNA was permanently attached to the membrane by UV illumination for 150 s (GS Gene Linker, Bio-Rad, USA). The hybridization probe was obtained by PCR. The primers were as follows: β-actin, forward primer 5’-CGTCTGGACCTGGCTGGCCGGGACC-3’ and reverse primer 5’-CTAGAAGCATTTGCGGTGGACGATG-3’; TFDP2, forward primer 5’-GGAGTCAGGCAAATGCTCTC-3’ and reverse primer 5’-CTGCCCTCAGTATCCCTCAC-3’; E2F3, forward primer 5’-AAGAGCAGGAGCAGAGAGATG-3’ and reverse primer 5’-TTTGACAGGCCTTGACACTG-3’. α-32P-labeled cDNA probes were synthesized using Primer-a-Gene random labeling Kit (Promega, USA). Hybridization was performed overnight in rolling bottles containing 8 ml of hybridization buffer. The membranes were washed and exposed to X-ray films (Fuji Films, Tokyo, Japan) at -70 °C for 24-48 h.
RESULTS
Atlas human cancer cDNA microarray expression profile
Using a cDNA expression microarray technique we established the expression profile of 588 genes selected from different areas in human hepatoma cell line HLE and normal liver tissues (Figure 1A, 1B). No signals were visible in the blank spots and negative control spots indicating that the Atlas human cancer array hybridization was highly specific. The intensity for housekeeping genes was similar at the same time indicating that the results were credible. GAPDH was used to normalize the intensities. The comparison results analyzed by AtlasImage software showed that there were 30 genes changed, 22 up-regulated and 8 down-regulated in Hepatoma cell line HLE versus normal liver tissues. In the test, the ratio one over the other ≥ 1.5 or the difference between two ≥ 10000 was considered as up-regulated genes (Table 1).
Figure 1.
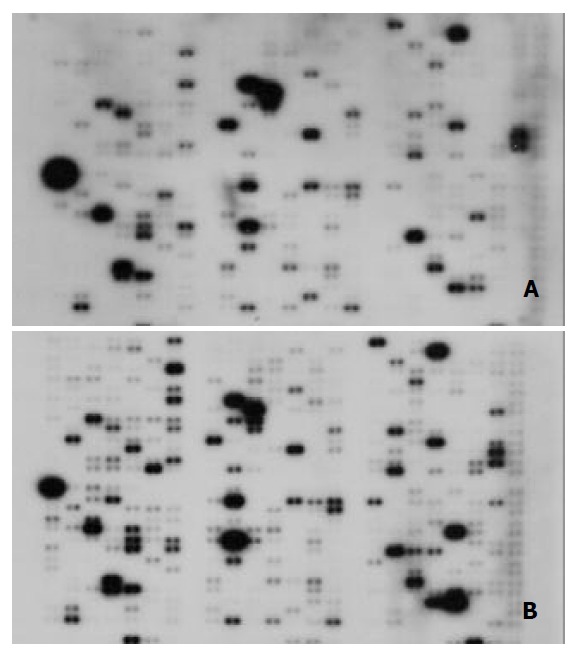
Parallel analyses of gene expression profiles in hu-man hepatoma cell line HLE and normal liver tissues. Atlas human cancer cDNA expression array (Clontech, USA) was hybridized with 32P-labeled cDNA probes in normal liver tis-sues (A) and human hepatoma cell line HLE (B).
Table 1.
Genes differentially expressed between hepatoma cell line HLE and normal liver tissues generated by AtlasImage software (Version 1.01a)
| Gene | Ratio | Difference | Protein/gene |
| F6k | 0.361 | -7908 | TDGF1 + TDGF2 + TDGF3 |
| B7g | 0.412 | -9606 | SKY (DTK) (TYRO3) (RSE) |
| A5b | 0.484 | -8262 | ERK activator kinase 1; MAPK/ERK kinase 1 (MEK1) |
| C5f | 0.487 | -8154 | Epidermal growth factor receptor substrate (eps15) |
| E5b | 0.504 | -14946 | Rho GDP-dissociation inhibitor 1 |
| D6c | 0.662 | -10998 | Semaphorin E |
| B7j | 0.664 | -12474 | Tyrosine-protein kinase ABL2 ; tyrosine kinase ARG (ABLL) |
| F5l | 0.732 | -12132 | Leukocyte interferon-inducible peptide |
| B2g | 1.237 | 10252 | TRAF-interacting protein (TRIP) |
| B3h | 1.241 | 10508 | Caspase-8 precursor; MACH; FLICE; (CAP4) (CASP8) |
| D5f | 1.363 | 12000 | CD9 |
| C7l | 1.381 | 14882 | Retinoic acid receptor gamma |
| A3i | 1.387 | 14088 | CDK inhibitor p19INK4d |
| C7m | 1.401 | 13224 | Retinoid X receptor beta (RXR-beta) |
| C6n | 1.444 | 11820 | Sex gene |
| D3e | 1.52 | 17176 | Vitronectin precursor; serum spreading factor; |
| D4b | 1.61 | 16212 | Integrin alpha8 |
| D2n | 1.692 | 19718 | TENASCIN-R |
| C1a | 1.804 | 15096 | DNA-dependent protein kinase (DNA-PK) |
| A4j | 2.008 | 17856 | Extracellular signal-regulated kinase 6 (ERK6) (ERK5) |
| D1b | 2.038 | 20026 | Byglycan |
| A7d | 2.06 | 18522 | Type II cytoskeletal 11 keratin; cytokeratin 1 (K1; CK 1); |
| A2k | 2.061 | 18084 | Cyclin C G1/S-specific |
| D4j | 2.178 | 27156 | Integrin beta7 |
| D4k | 2.252 | 28122 | Integrin beta8 |
| A7g | 2.257 | 13428 | Type II cytoskeletal 4 keratin; cytokeratin 4 (K4; CK4) |
| D1a | 2.31 | 29184 | Cartilage-specific proteoglycan core protein |
| (CSPCP); aggrecan1 | |||
| A5i | 2.418 | 10668 | E2F-3 |
| B4d | 2.474 | 11814 | Akt1; rac protein kinase alpha; protein kinase B; c-Akt |
| A5l | 4.256 | 37824 | DP2 dimerization partner of E2F |
Semi-quantitative RT-PCR
Twenty four paired tissues were performed for RT-PCR to verify accuracy and universality of the hybridization data. The RT-PCR results for 2 genes were consistent with hybridization data after normalization. Among the 24 paired tissues, the RT-PCR results of 2 genes were identical to the microarray results and the constituency was TFDP2 17/24, E2F3 16/24, respectively (Figure 2).
Figure 2.
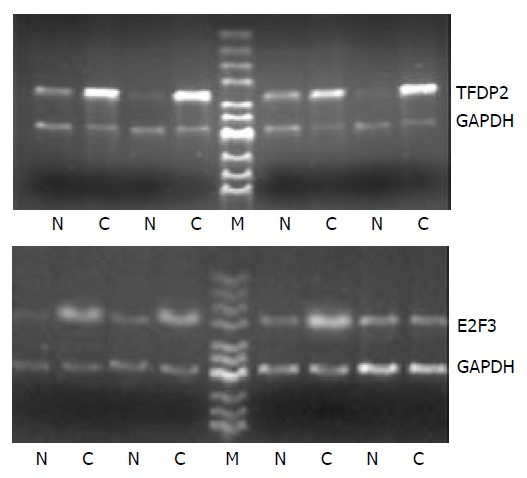
Partial semi-quantitative RT-PCR for 2 genes in 24 paired tissues. A total of 10 µl RT-PCR products were electro-phoresed on 2% agarose gel containing ethidium bromide. GAPDH was used as an internal control. (RT-PCR, reverse tran-scription polymerase chain reaction; N, adjacent normal liver tissue; C, human hepatocellular carcinoma tissue; GAPDH, glyceraldehyde-s-phosphate dehydrogenase; M, pUC Mix Maker).
Northern blot
Northern blot of four paired tissues were performed and verified the accuracy of the microarray. Among the 4 paired tissues, the northern blot results of 4 genes further meant that the Atlas human cancer cDNA microarray data were believable and comparable (Figure 3).
Figure 3.
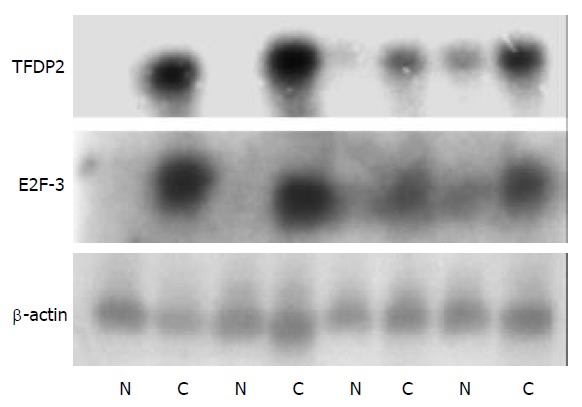
Northern blot analysis of 2 genes to confirm the Atlas human cancer cDNA expression array. Four paired cases were used to determine these genes expression patterns. Twenty µg RNA was analyzed on a 1.2% denaturing agrose gel and trans-ferred onto a nylon membrane. 32P-labeled cDNA probes for these genes were hybridized to the RNA-blotted membranes. After stringent washes, membranes were exposed to X-ray film overnight at -70 °C. The same membranes were rehybridized with human β-actin for an RNA loading control (N, adjacent normal liver tissue; C, human HCC tissue).
DISCUSSION
In this study, we explored the gene expression profiles in human hepatoma cell line HLE and normal liver tissues using Atlas human cancer array. cDNA array technology was used to examine simultaneously the expression of specific genes on a single hybridization. Although human genome projects have generated a large-scale sequence data for millions of genes, the biological functions of such genes remain to be deciphered. It is very important to define differential gene expression profiles of tumors and normal tissues before understanding the functional significance of specific gene products. Thus a systematic approach for examining large number of genes simultaneously is required. Microarray techniques have been developed in this conditions[23]. The gene expression profiles obtained from microarray technique was first reported in 1995[16]. It might be useful for tumor classification, elucidation of key factor in tumors, and identification of genes[24-27].
Several genes related to cell cycle regulators, growth regulators were abundantly up-regulated in our HCC samples. E2F-3 is a transcription factor that plays an important role during the cell cycle of proliferating cells, its products are rate limiting for initiation of DNA replication[28]. E2F-3 is able to activate transcription of E2F-responsive genes in a manner depending upon the presence of at least one functional E2F binding site[29,30]. TFDP2 gene is a member of the TFDP genes family of transcription factors. TFDP2 protein can form heterodimers with E2F family proteins in vivo. The E2F-TFDP transcription factors are major regulators of genes that are required for the progression of S-phase and they play a critical role in cell cycle regulation and differentiation[29]. TFDP2 may plays a major role in modulating the function of E2F in cell cycle regulation and oncogenesis. The retinoblastoma tumor suppressor protein has been shown to induce growth arrest by binding to E2F-TFDP and repressing its activity[31]. The up-regulation of E2F-3 and TFDP2 may play an important role in HCC cell proliferation.
Protein kinase B (PKB)/Akt is a growth-factor-regulated serine/threonine kinase which contains a pleckstrin homology domain[32]. Activated PKB/Akt provides a survival signal that protects cells from apoptosis induced by various stresses, and also mediates a number of metabolic effects of insulin[33]. The up-regulation of Akt1 may have a great help for tumor cell to survive from apoptosis.
In our study, genes related to DNA repair and recombination was up-regulated in HLE, which indicates that the generative ability of tumor cell was stronger than that of normal hepatocytes. DNA-dependent protein kinase (DNA-PK) is a nuclear protein serine/threonine kinase that is activated by DNA double strand breaks (DSBs). It is a component of the DNA DSB repair apparatus, and cells deficient in DNA-PK are hypersensitive to ionizing radiation and radio-mimetic drugs[34]. DNA-PK may have roles in controlling transcription, apoptosis, and the length of telomeric chromosomal ends. The DNA-PK complex is regulated in a cell cycle-dependent manner, with peaks of activity found at the G1/early S phase and again at the G2 phase in wild-type cells[35].
Integrins are a major family of cell adhesion molecules involved in cell-cell and cell-extracellular matrix interactions. Integrins are suggested to be involved in many different biological processes such as growth, differentiation, migration, and cell death. The integrin alpha 8 beta 1 has been reported to bind to fibronectin, vitronectin, tenascin-C and osteopontin in cell adhesion or neurite outgrowth assays[36,37]. Integrins are heterodimeric cell adhesion proteins connecting the extracellular matrix to the cytoskeleton and transmitting signals in both directions[38]. The up-regulation of Integrin may play a role in HCC metastasis.
In conclusion, our study demonstrates the cDNA array is a powerful tool to explore gene expression profiles in cancer. The genes described in this study provide valuable resources not only for basic research, such as molecular mechanism of carcinogenesis, progression and prognosis, but also for clinical application, such as development of new diagnostic markers, identification of therapeutic intervention in human HCC.
Footnotes
Supported by China Key Program on Basic Research, No.Z-19-01-01-02; Chinese Climbing Project, No.18; Youth Natural Scientific Foundation of Heilongjiang Province and Harbin, No.QC01C11
Edited by Ren SY
References
- 1.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 2.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu W, Lin XB, Qian JM, Ji ZL, Jiang Z. Ultrasonic aspiration hepatectomy for 136 patients with hepatocellular carcinoma. World J Gastroenterol. 2002;8:763–765. doi: 10.3748/wjg.v8.i4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang ZY, Sun FX, Tian J, Ye SL, Liu YK, Liu KD, Xue Q, Chen J, Xia JL, Qin LX, et al. Metastatic human hepatocellular carcinoma models in nude mice and cell line with metastatic potential. World J Gastroenterol. 2001;7:597–601. doi: 10.3748/wjg.v7.i5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu LX, Jiang HC, Piao DX. Radiofrequence ablation of liver cancers. World J Gastroenterol. 2002;8:393–399. doi: 10.3748/wjg.v8.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208–215. doi: 10.3748/wjg.v7.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao WH, Ma ZM, Zhou XR, Feng YZ, Fang BS. Prediction of recurrence and prognosis in patients with hepatocellular carcinoma after resection by use of CLIP score. World J Gastroenterol. 2002;8:237–242. doi: 10.3748/wjg.v8.i2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang HC, Liu LX, Piao DX, Xu J, Zheng M, Zhu AL, Qi SY, Zhang WH, Wu LF. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002;8:624–630. doi: 10.3748/wjg.v8.i4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yip D, Findlay M, Boyer M, Tattersall MH. Hepatocellular carcinoma in central Sydney: a 10-year review of patients seen in a medical oncology department. World J Gastroenterol. 1999;5:483–487. doi: 10.3748/wjg.v5.i6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74–78. doi: 10.3748/wjg.v8.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang WH, Zhu SN, Lu SL, Huang YL, Zhao P. Three-dimensional image of hepatocellular carcinoma under confocal laser scanning microscope. World J Gastroenterol. 2000;6:344–347. doi: 10.3748/wjg.v6.i3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61–65. doi: 10.3748/wjg.v6.i1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sithinamsuwan P, Piratvisuth T, Tanomkiat W, Apakupakul N, Tongyoo S. Review of 336 patients with hepatocellular carcinoma at Songklanagarind Hospital. World J Gastroenterol. 2000;6:339–343. doi: 10.3748/wjg.v6.i3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, Zhou XD, Ye SL. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001;7:28–32. doi: 10.3748/wjg.v7.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 17.DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M, Chen Y, Su YA, Trent JM. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet. 1996;14:457–460. doi: 10.1038/ng1296-457. [DOI] [PubMed] [Google Scholar]
- 18.Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M. Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol. 2002;8:580–585. doi: 10.3748/wjg.v8.i4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Gan L, Jeffery E, Gayle M, Gown AM, Skelly M, Nelson PS, Ng WV, Schummer M, Hood L, et al. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229:101–108. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 20.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- 21.Khan J, Simon R, Bittner M, Chen Y, Leighton SB, Pohida T, Smith PD, Jiang Y, Gooden GC, Trent JM, et al. Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res. 1998;58:5009–5013. [PubMed] [Google Scholar]
- 22.Lu J, Liu Z, Xiong M, Wang Q, Wang X, Yang G, Zhao L, Qiu Z, Zhou C, Wu M. Gene expression profile changes in initiation and progression of squamous cell carcinoma of esophagus. Int J Cancer. 2001;91:288–294. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1063>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Kallioniemi OP. Biochip technologies in cancer research. Ann Med. 2001;33:142–147. doi: 10.3109/07853890109002069. [DOI] [PubMed] [Google Scholar]
- 24.Liu LX, Jiang HC, Liu ZH, Zhou J, Zhang WH, Zhu AL, Wang XQ, Wu M. Integrin gene expression profiles of human hepatocellular carcinoma. World J Gastroenterol. 2002;8:631–637. doi: 10.3748/wjg.v8.i4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan J, Saal LH, Bittner ML, Chen Y, Trent JM, Meltzer PS. Expression profiling in cancer using cDNA microarrays. Electrophoresis. 1999;20:223–229. doi: 10.1002/(SICI)1522-2683(19990201)20:2<223::AID-ELPS223>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Hu YC, Lam KY, Law S, Wong J, Srivastava G. Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC. Clin Cancer Res. 2001;7:2213–2221. [PubMed] [Google Scholar]
- 27.Selaru FM, Zou T, Xu Y, Shustova V, Yin J, Mori Y, Sato F, Wang S, Olaru A, Shibata D, et al. Global gene expression profiling in Barrett's esophagus and esophageal cancer: a comparative analysis using cDNA microarrays. Oncogene. 2002;21:475–478. doi: 10.1038/sj.onc.1205111. [DOI] [PubMed] [Google Scholar]
- 28.Leone G, DeGregori J, Yan Z, Jakoi L, Ishida S, Williams RS, Nevins JR. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 1998;12:2120–2130. doi: 10.1101/gad.12.14.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moberg K, Starz MA, Lees JA. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Chellappan SP. Cloning and characterization of human DP2, a novel dimerization partner of E2F. Oncogene. 1995;10:2085–2093. [PubMed] [Google Scholar]
- 31.Zhang Y, Venkatraj VS, Fischer SG, Warburton D, Chellappan SP. Genomic cloning and chromosomal assignment of the E2F dimerization partner TFDP gene family. Genomics. 1997;39:95–98. doi: 10.1006/geno.1996.4473. [DOI] [PubMed] [Google Scholar]
- 32.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 33.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 34.Jackson SP. DNA-dependent protein kinase. Int J Biochem Cell Biol. 1997;29:935–938. doi: 10.1016/s1357-2725(97)00006-x. [DOI] [PubMed] [Google Scholar]
- 35.Lee SE, Mitchell RA, Cheng A, Hendrickson EA. Evidence for DNA-PK-dependent and -independent DNA double-strand break repair pathways in mammalian cells as a function of the cell cycle. Mol Cell Biol. 1997;17:1425–1433. doi: 10.1128/mcb.17.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denda S, Reichardt LF, Müller U. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol Biol Cell. 1998;9:1425–1435. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denda S, Müller U, Crossin KL, Erickson HP, Reichardt LF. Utilization of a soluble integrin-alkaline phosphatase chimera to characterize integrin alpha 8 beta 1 receptor interactions with tenascin: murine alpha 8 beta 1 binds to the RGD site in tenascin-C fragments, but not to native tenascin-C. Biochemistry. 1998;37:5464–5474. doi: 10.1021/bi9727489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]


