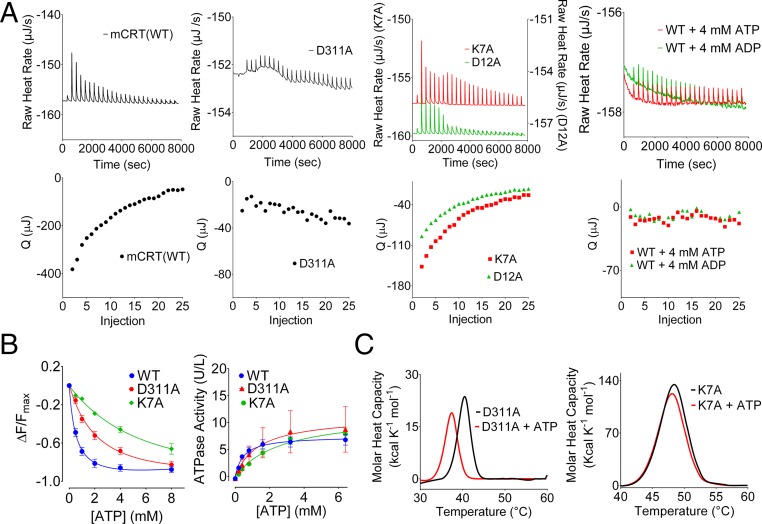
Fig. 3.
Influences of nucleotides on calcium binding and of a calcium-binding site mutant on nucleotide binding. (A) Representative ITC thermograms depicting the binding of calcium to the high-affinity calcium-binding sites of the indicated calreticulin constructs in the absence or presence of 4 mM ATP or ADP. Plots show raw titration curves (Upper) and the corresponding curve fits (Lower). Data are representative of two to four [mCRT mutants: mCRT(WT) + ATP and mCRT(WT) + ADP] or nine [mCRT(WT)] independent experiments. Q, area of the indicated injection peak. (B, Left) Dose-dependent quenching of the Trp fluorescence of the indicated calreticulin constructs in the presence of varying concentrations of ATP. (B, Right) Michaelis–Menten kinetic plots and associated best global fit showing the rate of ATP hydrolysis by the indicated calreticulin constructs. Data show mean ± SEM. Binding and kinetic constants, along with data replicates, are shown in Table 1. ΔF, change in the intrinsic Trp fluorescence peak value relative to the ATP-free state; Fmax, intrinsic Trp fluorescence peak value in the absence of ATP. (C) Representative DSC thermograms (of three to four independent experiments) showing ATP-induced thermostability changes for mCRT(D311A) (Left) and mCRT(K7A) (Right).