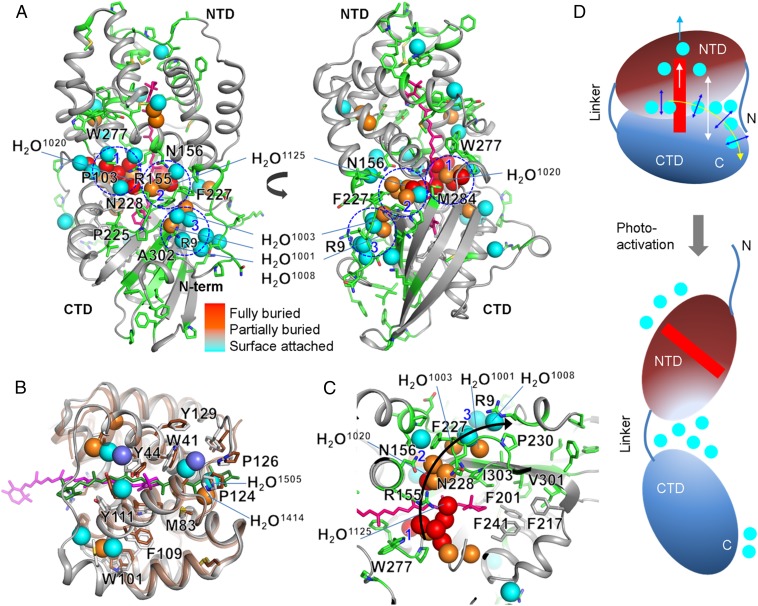
Fig. 4.
Water network for carotenoid signaling in OCP. (A) Modified residues and the carotenoid are represented by green and pink sticks, respectively, on the X-ray crystal structure of OCPO from Synechocystis (PDB ID code 3MG1). The spheres represent water molecules, which are either conserved (labeled) or located at approximately the same position (within 0.5 Å) in both the crystal structure of A. maxima (4) and Synechocystis OCP (5). The coloring of the waters indicates their depth from the surface of the OCP (see Fig. S1 C–E for additional details). Three major water clusters, at the minor and major interfaces, are marked by blue dashed lines and numbered. These waters potentially form internal H-bonding contacts with side chains, amide backbone, and other water molecules (Fig. S1F and Table S6). Residues that undergo the highest degree of solvent accessibility change and are important for the H-bonding network at the major and minor interface are labeled. (B) Superimposed structure of the constitutively active RCP (protein in brown, carotenoid in green, buried water as violet spheres) (9) and Synechocystis OCPO (gray, carotenoid in pink, conserved and buried water colored as in A) (5). Residues that are proposed to stabilize the carotenoid in the activated state are labeled. (C) Proposed signal propagation pathway from the carotenoid through the water-side chain H-bonding network to the protein surface that facilitates carotenoid shift, dissociation of NTD-CTD, and detachment of the N-terminal helix from CTD. (D) Schematic of the OCPO and OCPR showing regions with the largest conformational rearrangement associated with the change in H-bonding network and water rearrangements.