Abstract
AIM: Bacillary dysentery caused by Shigella flexneri is still a threat to human health. Of four invasion plasmid antigen proteins (IpaA, B, C and D), IpaC plays an important role in the pathogenicity of this pathogen. The purpose of this study was to investigate the proteins interacting with IpaC in the host cell during the pathogenic process of this disease.
METHODS: By applying two-hybrid system, the bait plasmid containing ipaC gene was constructed and designated pGBKT-ipaC. The bait plasmid was transformed AH109, and proved to express IpaC and then HeLa cDNA library plasmids were introduced into the above transformed AH109. The transformation mixture was plated on medium lacking Trp, Leu, and His in the initial screen, then restreaked on medium lacking Trp, Leu, His and Ade. Colonies growing on the selection medium were further assayed for β-galactosidase activity. BLAST was carried out in the database after sequencing the inserted cDNA of the positive library plasmid.
RESULTS: Among the 2 × 106 transformants, 64 positive clones were obtained as determined by activation of His, Ade and LacZ reporter genes. Sequence analysis revealed that cDNA inserts of two colonies were highly homologous to a known human protein, RanBPM.
CONCLUSION: These results provide evidence that IpaC may be involved in the invasion process of S. flexneri by interacting with RanBPM, and RanBPM is most likely to be the downstream target of IpaC in the cascade events of S. flexneri infection.
INTRODUCTION
Shigella is a Gram-negative bacterium responsible for intestinal diseases ranging from mild watery diarrhea to bacillary dysentery in humans. The severe forms of shigellosis are due to colonization and destruction of the colonic mucosa by this invasive pathogen. The infectious potential of Shigella is very high, since as few as 100 microorganisms administered orally are sufficient to cause dysentery in volunteers. Each year, at least a billion cases of diarrheal diseases account for about three million deaths. In the developing world, children under 5 years of age are the most susceptible victims, with over half a million deaths occurred annually worldwide[1].
The phenotype which is essential to the pathogenicity of S. flexneri is encoded by a 31 kb sequence[2,3] located on the 200 kb large virulence plasmid[4,5]. One locus in this fragment, composed of the ipa operon (invasion plasmid antigen), is necessary to encode and secrete the effectors, the Ipa proteins or invasins. The ipa operon encodes four secreted proteins: IpaB(62 kDa), IpaC(42 kDa), IpaD(37 kDa) and IpaA(70 kDa), which elicit the formation of the entry focus via localized actin polymerization[6]. The Ipa proteins are rapidly secreted from S. flexneri when the bacterium comes into contact with epithelial cells[7-10]. Following their secretion, IpaB and IpaC are found as part of a protein complex and this complex is absolutely required for entry into epithelial cells[7,11,12]. Latex beads coated with anti-IpaC antibodies have been used to recover the extracellular Ipa complex containing IpaB and IpaC, and were shown to be internalized in HeLa cells through the formation of membrane ruffles similar to those induced upon bacterial entry[13]. These results have led to the proposal that IpaB and IpaC play major roles in the entry of Shigella into epithelial cells.
Although IpaB is undoubtedly important for S. flexneri invasion[14-17], a great deal of foci were shifted to IpaC as a potential effector of S. flexneri invasion when purified IpaB was shown not to possess in vitro membranolytic activity[11], and purified IpaC was demonstrated to have such activities consistent with its contribution to invasion[18]. Effector-related functions that have been demonstrated for purified IpaC include: enhanced invasion of cultured cells by S. flexneri at nanomlar IpaC concentrations[16,19]; induced uptake of virulence plasmid-cured at micromolar IpaC concentrations[24]; association with model phospholipid membranes[16,21]; and triggering of cytoskeletal changes in cultured cells[22,27]. Additional activities associated with IpaC also include in vitro reconstitution into complexes with IpaB, which may promote the uptake of non-invasive strains of Escherichia coli[12,19].
Shigellosis provides a model to study how a pathogenic microorganism can subvert an integrated defense barrier and interact with the host cells, which in turn facilitates invasion at the early stage of the process. A major challenge is to understand the role of Ipa proteins in entry into epithelial cells. In order to investigate the mechanism of IpaC in the entry process, two-hybrid system was exploited to identify the proteins in host cells that interacted with IpaC invasin.
MATERIALS AND METHODS
Materials
The strains and plasmids used in this study are listed in Table 1.
Table 1.
Strains and plasmids
| Relevant characteristics | Source | |
| Strains | ||
| S. flexneri 2a, 2457T | Wild type, Nalr | Maurelli AT |
| E. coli DH5α | SupE44, ΔlacU169 (Φ80 lacZΔM15), hsdR17, recA1, endA1, gyrA96, thi-1, relA1, Nalr | Our lab |
| S. cerevisiae AH109 | MATa, trp1-901, leu2-3, ura3-52, his3-200, gal4Δ, gal80Δ, LYS2::GAL1UAS-GAL1TATA-HIS3, GAL2UAS-GAL2TATA-ADE2, URA3::MEL1UAS-MEL1TATA-LacZ | Clontech |
| Plasmids | ||
| pGBKT7 | GAL4 DNA-BD, TRP1, c-Myc epitope tag, Kmr Clontech | |
| pGBKT-ipaC | GAL4 DNA-BD fusion of IpaC, TRP1, c-Myc epitope tag, Kmr | This study |
| pACT2 | GAL4 DNA-AD, HA epitope, LEU2, Apr | Clontech |
| pLAM5'-1 | GAL4 DNA-BD fusion of the human lamin C, TRP1, a false-positive detection plasmid, Apr | Clontech |
| p1 | A positive library plasmid | This study |
Human HeLa MATCHMAKER cDNA library (HL4048AH), c-Myc monoclonal antibody, YPD medium, minimal SD base and DO supplement medium were purchased from Clontech. Peroxidase-conjugated goat anti-mouse IgG was from Santa Cruz. Mini- and Megapreps plasmid kits were obtained from Promega. Restriction endonucleases and T4 DNA ligase were purchased from New England Biolabs. Taq DNA polymerase, deoxyribonucleotides, and PCR fragment recovery kit were from TaKaRa. Primers for the 5’ and 3’ ends of ipaC gene, P1 and P2, were synthesized in our lab.
Methods
Growth and maintenance of strains Shigella flexneri 2a was grown at 37 °C with constant stirring in trypticase soy broth(TSB). Prior to use, the bacteria were streaked onto trypticase soy agar (TSA) containing 0.025% Congo red so that colonies binding the dye could be selected. Bacteria that had lost the invasion plasmid were not able to bind this dye and thus appeared white in the presence of Congo red. Yeast strain AH109 was grown at 30 °C with vigorous shaking in YPAD. Antibiotics were used at the following concentrations: ampicillin (Ap), 100 mg•l-1; kanamycin (Km), 50 mg•l-1; nalidixic acid (Nal), 40 mg•l-1.
Construction of bait plasmid Isolation of plasmid and all other molecular biology procedures were carried out according to the standard published procedures[22]. ipaC gene was amplified by PCR in a standard 100-μL reaction containing 2.5 mM MgCl2, 0.25 mM of each dNTP, 100 pmol of P1 and P2 primers, 10 μL boiled S. flexneri, and 5 U Taq DNA polymerase. Reactions were allowed to proceed in a Perkin-Elmer 2400 thermal cycler programmed for 30 cycles (94 °C, 30 s; 56 °C, 30 s; and 72 °C, 90 s) with one additional cycle for 7 min at 72 °C. PCR product was purified by agarose gel electrophoresis, digested by Nco I and Sal I, then ligated into the pGBKT7 plasmid digested by Nco I and Sal I. The ligation products were then transformed into E.coli DH5α. The insert of ipaC gene was then confirmed by PCR using the conditions described above. To confirm if the ipaC gene was fused in frame to the coding sequence of the GAL4 DNA-binding domain, the plasmids were subjected to double-stranded DNA sequencing with T7 sequencing primer according to the manufacturer's specifications.
Yeast transformation The lithium acetate (LiAc)-mediated method was performed to transform DNA into yeast as described in the reference[23]. In brief, the yeast competent cells were prepared and suspended in a LiAc solution with the plasmids DNA to be transformed, along with excess carrier DNA. Polyethylene glycol (PEG) with an appropriate amount of LiAc was then added and the mixture of DNA and yeast was incubated at 30 °C. After incubation, DMSO was added and the cells were heat shocked at 42 °C. The cells were then plated on the appropriate medium to select transformants. The amount of plasmids used for transformation was 0.1 μg except that 50 μg library plasmid was used.
SDS-PAGE and western blot analysis Extraction of yeast protein sample was carried out by using urea/SDS method as described in reference[24]. SDS-PAGE was performed using the standard procedure of Laemmli[22]. The samples were resolved on a 12% polyacrylamide gel. The samples could be stained with Coomassie brilliant blue R250, or the proteins electroblotted to NC membranes for western blot analysis using a Bio-Rad Transbolt Semi-dry Blotter according to the manufacturer's instructions. Western blot analysis was performed as previously described[22,25]. Briefly, the membranes were blocked following protein transfer by incubation in TBS (50 mM Tris-HCl pH7.4, 5% BSA, 500 mM NaCl) and then incubated with c-Myc monoclonal antibody diluted in TBS containing 0.1% Tween 20 (v/v). After several rinses in the same buffer, the membrane was incubated with HRP-conjugated secondary antibody in the same buffer. The membrane was then rinsed in TBS containing 10 mM EDTA, 0.5 M NaCl , and 0.1% Tween 20 (w/v). Blots were developed using TBS (50 mM Tris-HCl pH7.4, 5% BSA, 500 mM NaCl) containing 0.7 mg/mL 3, 3'-diaminobenzidine (DAB) and 0.003% H2O2, finally terminated with 2M H2SO4.
Two-hybrid strategy Before the screening process, intrinsic activation function of the bait was tested as demonstrated by a bait-testing assay. To perform the two-hybrid screening, the bait plasmid pGBKT-ipaC was transformed into the yeast strain AH109. The HeLa cDNA libraries were extracted from E.coli and introduced into the AH109 transformed with the bait. Cells were first spread on plates lacking Leu, Trp and His, and containing 1mM 3-aminotriazole to select colonies by prototrophy for histidine. The His+ colonies were streaked on selective plates lacking Leu, Trp, His and Ade. The His+, Ade+ colonies were assayed for expression of β-galactosidase by an X-Gal overlay assay, and blue colonies were streaked twice on selective plates. After that, individual His+, Ade+, LacZ+ colonies were cultured in liquid SD synthetic medium. Yeast DNA was recovered and transformed into E. coli DH5α by electroporation. The insert carried by the prey plasmid in each of the selected clones was amplified by PCR and grouped. Representative plasmids from each group were retransformed back into yeast to test their interaction with IpaC. The cDNA inserts were subjected to sequencing.
RESULTS
Construction of bait plasmid
PCR primers for the 5’ and 3’ ends of ipaC gene were designed as follows based on its published sequences[26]: P1, 5'-CGGCCATGGTAATAGAACTGATGTTGC-3' containing an Nco I restriction site and 18 bases of the 5’ end of ipaC gene; and P2, 5'-GCGTCGACTTAAGCTCGAATGTTAC-3' containing a Sal I restriction site and 18 bases of the 3' end of ipaC gene. After amplification of ipaC gene by PCR, there was a band of about 1.1 kb by agarose gel electrophoresis (data not shown). The fragment was purified, digested by Nco I and Sal I, and cloned into the Nco I and Sal I sites of pGBKT7. The resulting plasmid was designated as pGBKT-ipaC (Figure 1). Sequencing revealed that the ipaC gene was fused in the correct reading frame with the coding sequence of GAL4 DNA-binding domain (data not shown).
Figure 1.
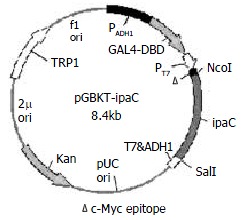
Construction of recombinant plasmid expressing IpaC.
Expression of IpaC in yeast
SDS-PAGE and western blotting were carried out with the protein extracted from yeast AH109, AH109 transformed with pGBKT7 and pGBKT-ipaC (Figure 2). An approximate 20 kDa GAL4 DNA binding domain containing c-Myc epitope tag was expressed in AH109 transformed with pGBKT7, while a 62kDa peptide was expressed in AH109 transformed with pGBKT-ipaC. This showed that IpaC could be expressed in frame with the GAL4 DNA binding domain and the bait plasmid could be used in two-hybrid screening.
Figure 2.
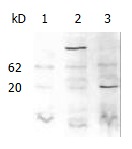
Western blot analysis of the fused expression of IpaC with c-Myc monoclonal antibody as a primary antibody. Lane 1, AH109; lane 2, AH109 transformed with pGBKT-ipaC; lane 3, AH109 transformed with pGBKT7.
Two-hybrid screening
In order to identify whether the fused bait protein could activate the expression of reporter genes, yeast containing the bait plasmid (pGBKT-ipaC) and yeast containing the empty vector of library (pACT2) were tested as demonstrated by a bait-testing assay (Table 2). The results indicated that IpaC was devoid of transcriptional activity and could be used as bait in a yeast two-hybrid assay.
Table 2.
Detection of transcriptional activity of bait fusion protein in AH109
| Tranformed plasmids | Growth on SD His- | X-Gal |
| pGBKT-ipaC | - | white |
| pGBKT-ipaC + pACT2 | - | white |
The bait plasmid and cDNA library were sequentially transformed into yeast strain AH109. Initial screening of 2 × 106colonies of a human HeLa MATCHMAKER cDNA library identified 92 clones that showed specific activation of His reporter gene. Further testing of the specificity of interaction screening on selective media lack of Trp, Leu, His, Ade and by β-galactosidase assay showed that only 64 colonies interacted specifically with the bait BD-IpaC protein, but not with fusion proteins between BD and human lamin C (Table 3).
Table 3.
Verification of the interaction in positive clones
| Tranformed plasmids | β-Gal activity | |
| 1 | p1 | white |
| 2 | p1 + pGBKT7 | white |
| 3 | p1 + pLAM5’-1 | white |
| 4 | p1 + pGBKT-ipaC | blue |
Sequence analysis of the clones revealed that two independent colonies (p1 and p25) contained the same gene. A database search using the BLAST program showed that this gene encoded the protein which had as much as 99% homology with RanBPM (GenBank accession number AB055311). The two library colonies isolated from the yeast-two hybrid assay contained amino acid sequences that were upstream of the BPM55 start codon, but were within the coding region of BPM90. The resulting product of p1 contained 584 C-terminal amino acids of RanBPM, which fused in the frame with the GAL4 activation domain.
DISCUSSION
An important step in S. flexneri infection is bacterial invasion of colonic epithelial cells, as characterized by host cytoskeletal rearrangements at the site of bacterial contact[27]. These localized changes in the host cytoskeleton lead to the formation of filopodia that combine and trap the pathogen within a membrane-bound vacuole that is rapidly lysed, thereby providing the bacterium with access to the host cell cytoplasm[6]. Evidence indicates that IpaC is necessary and sufficient to promote the formation of filopodial extensions localized at the edge of fibroblastic cells[21]. These extensions appear within seconds after exposure of permeabilized cells to IpaC. The data presented above and those from other laboratories indicate that IpaC proteins play an important role in this process.
Current understanding of the role of S. flexneri Ipa proteins in epithelial cell invasion is primarily based on deletion mutagenesis, genetic complementation, and immunological analyses[12-14,28,29]. To better understand the roles of the Ipa proteins in the pathogenesis of shigellosis, it is necessary to isolate proteins that interact with Ipa proteins in host cells. The two-hybrid system is an effective genetic method to identify protein-protein interaction and has been increasingly used[30,31]. Therefore, we exploited a two-hybrid screen to identify the IpaC-interacting protein in host cells. The foci of infection of this pathogen are human colonic epithelial cells, so a library prepared from them should be used in our screening. Unfortunately, it is not commercially available. The mechanism of entry of Shigella into cells has been studied extensively in cultured cell lines. Of these cell lines, Hela was derived from human epithelial cells and has been frequently used in the invasion of S. flexneri[32-35]. Therefore a HeLa cDNA library was used instead in our screening. Two library colonies that were isolated in the yeast-two hybrid assay contained amino acid sequences with high homology to RanBPM.
RanBPM was originally identified by its interaction with Ran, a small Ras-like GTPase. Ran shuttles between the nucleus and the cytoplasm to complete its GTPase cycle, carrying out nucleocytoplasmic transport of macromolecules and inducing microtubule self-assembly by interacting with distinct Ran binding protein[36,37]. RanBPM was initially identified as a 55 kD protein (BPM55) which contains 500 amino acids[37]. A subsequent report has shown that BPM55 is a truncated protein and the full-sized RanBPM is a 90 kD protein (BPM90) which contains 729 amino acids[38]. Here we demonstrated that the function domain within BPM90 was able to interact with IpaC in vivo. RanBPM was predominantly localized both in the nucleus and in the cytoplasmic region surrounding the centrosome[38]. When truncated RanBPM was overexpressed in green monkey kidney COS7 cells, the multiple spots which were colocalized with γ-tubulin were formed and acted as ectopic microtubule nucleation sites, resulting in a reorganization of microtubule network[37]. The function of BPM90 is still unclear, but it has recently been linked to the Ras/Erk signaling pathway[39].
Actin filaments and microtubule (MT) arrays have been regarded as constituting separate cytoskeletal systems with distinct functions. However, it has become clear that the cell's system of cytoskeletal filaments and its network of signaling pathways are intimately linked and function cooperatively to generate a cell phenotype tailored to the immediate conditions of the cell[40]. When a signal occurs, the structural responses driven by the cytoskeleton are usually complex, such as establishing new axes of polarity, making and breaking contacts, moving or dividing, especially generating protrusions as observed in the entry process of S. flexneri.
Taken the data presented above together, we therefore become greatly interested in possible involvement of IpaC in the signaling cascade resulting in massive cytokeletal rearrangements (Figure 3).
Figure 3.
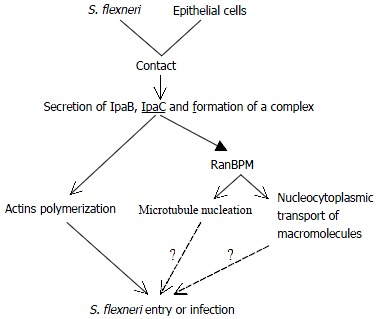
A schematic diagram of possible IpaC signaling cascade in S. flexneri entry.
The early step of infection is the invasion of epithelial cells of the colon. As bacteria contact the cell surface, Ipa invasins are secreted through the specialized secretory apparatus, two of which (IpaB and IpaC) form a complex. IpaC has hydrophobic regions that form transmembrane helices and insert into cell membrane[41]. This complex constitutes the primary effector of Shigella entry and is able to activate entry via its interaction with the host cell membrane. Then IpaC stimulates localized accumulation of filamentous actin at the site of bacterial contact. Meanwhile IpaC also leads to microtubule nucleation and/or nucleocytoplasmic transport of macromolecules possibly by interacting with RanBPM. So it is likely that IpaC elicits major rearrangements of host cell cytoskeleton, and these cytoskeletal filaments function cooperatively and form bundles supporting the membrane projections which achieve bacterial entry. Both the pathogen and the host cell actively contribute to this process.
This is the first report that IpaC interacts with RanBPM identified by two-hybrid screening. This information provides an important step in studying the protein-protein interaction that occurs in the host cells during initiation of Shigella pathogenesis. Although our understanding of the mechanisms that govern Shigella entry and actin-based intracellular motility has improved at a considerable pace, many questions remain open. In particular, it will be interesting to establish various connections between signaling pathways involved in Shigella entry and responses linked to the ipa proteins. Understanding how these responses integrate together and act in a concerted manner to induce bacterial entry will represent another exciting field of investigations.
ACKNOWLEDGMENTS
We would like to thank Prof. Qi-Nong Ye for his technical assistance and helpful discussions. We also thank Prof. Jian-Guang Zhou, Dr. Hao Zhang for their support in this project. We acknowledge the technical assistance of De-Hui He, Kun Hu and Zhi-Yong Gao.
Footnotes
Supported by the State Education Commission Research Foundation for Returned Overseas Chinese Scholars Abroad (1997) 436
Edited by Wu XN
References
- 1.Lindberg AA, Pál T. Strategies for development of potential candidate Shigella vaccines. Vaccine. 1993;11:168–179. doi: 10.1016/0264-410x(93)90014-o. [DOI] [PubMed] [Google Scholar]
- 2.Maurelli AT, Baudry B, d'Hauteville H, Hale TL, Sansonetti PJ. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasakawa C, Kamata K, Sakai T, Makino S, Yamada M, Okada N, Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988;170:2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sansonetti PJ, Kopecko DJ, Formal SB. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sansonetti PJ, Hale TL, Dammin GJ, Kapfer C, Collins HH, Formal SB. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983;39:1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerc P, Sansonetti PJ. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsot C, Ménard R, Gounon P, Sansonetti PJ. Enhanced secretion through the Shigella flexneri Mxi-Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol Microbiol. 1995;16:291–300. doi: 10.1111/j.1365-2958.1995.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 8.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ménard R, Sansonetti P, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Disulfide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc Natl Acad Sci U S A. 1995;92:4927–4931. doi: 10.1073/pnas.92.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ménard R, Sansonetti P, Parsot C, Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 12.Ménard R, Prévost MC, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci U S A. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ménard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.High N, Mounier J, Prévost MC, Sansonetti PJ. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoudy A, Mounier J, Aruffo A, Ohayon H, Gounon P, Sansonetti P, Tran Van Nhieu G. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell Microbiol. 2000;2:19–33. doi: 10.1046/j.1462-5822.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 16.Tran N, Serfis AB, Osiecki JC, Picking WL, Coye L, Davis R, Picking WD. Interaction of Shigella flexneri IpaC with model membranes correlates with effects on cultured cells. Infect Immun. 2000;68:3710–3715. doi: 10.1128/iai.68.6.3710-3715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran Van Nhieu G, Bourdet-Sicard R, Duménil G, Blocker A, Sansonetti PJ. Bacterial signals and cell responses during Shigella entry into epithelial cells. Cell Microbiol. 2000;2:187–193. doi: 10.1046/j.1462-5822.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 18.Marquart ME, Picking WL, Picking WD. Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect Immun. 1996;64:4182–4187. doi: 10.1128/iai.64.10.4182-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis R, Marquart ME, Lucius D, Picking WD. Protein-protein interactions in the assembly of Shigella flexneri invasion plasmid antigens IpaB and IpaC into protein complexes. Biochim Biophys Acta. 1998;1429:45–56. doi: 10.1016/s0167-4838(98)00213-1. [DOI] [PubMed] [Google Scholar]
- 20.De Geyter C, Vogt B, Benjelloun-Touimi Z, Sansonetti PJ, Ruysschaert JM, Parsot C, Cabiaux V. Purification of IpaC, a protein involved in entry of Shigella flexneri into epithelial cells and characterization of its interaction with lipid membranes. FEBS Lett. 1997;400:149–154. doi: 10.1016/s0014-5793(96)01379-8. [DOI] [PubMed] [Google Scholar]
- 21.Tran Van Nhieu G, Caron E, Hall A, Sansonetti PJ. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 1999;18:3249–3262. doi: 10.1093/emboj/18.12.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd editor. New York: Cold Spring Harbour Press; 1989. [Google Scholar]
- 23.Gietz D, St Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Printen JA, Sprague GF. Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics. 1994;138:609–619. doi: 10.1093/genetics/138.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burnette WN. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 26.Venkatesan MM, Buysse JM, Kopecko DJ. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988;85:9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labrec EH, Schneider H, Magnani TJ, Formal SB. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964;88:1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasakakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J Bacteriol. 1993;175:2334–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills JA, Buysse JM, Oaks EV. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun. 1988;56:2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fields S, Sternglanz R. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 1994;10:286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 31.Li K, Wang L, Cheng J, Zhang LX, Duan HJ, Lu YY, Yang JZ, Liu Y, Hong Y, Xia XB, et al. Screening and cloning of gene of hepatocyte protein 1 interacting with HCV core protein. Shijie huaren xiaohua zazhi. 2001;9:1379–1383. [Google Scholar]
- 32.Skoudy A, Nhieu GT, Mantis N, Arpin M, Mounier J, Gounon P, Sansonetti P. A functional role for ezrin during Shigella flexneri entry into epithelial cells. J Cell Sci. 1999;112(Pt 13):2059–2068. doi: 10.1242/jcs.112.13.2059. [DOI] [PubMed] [Google Scholar]
- 33.Tran Van Nhieu G, Ben-Ze'ev A, Sansonetti PJ. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J. 1997;16:2717–2729. doi: 10.1093/emboj/16.10.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pál T, Hale TL. Plasmid-associated adherence of Shigella flexneri in a HeLa cell model. Infect Immun. 1989;57:2580–2582. doi: 10.1128/iai.57.8.2580-2582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong QP. Pathogenic effects of Opolysaccharide from Shigella flexneri strain. World J Gastroenterol. 1999;5:245–248. doi: 10.3748/wjg.v5.i3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bischoff FR, Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991;88:10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura M, Masuda H, Horii J, Kuma Ki, Yokoyama N, Ohba T, Nishitani H, Miyata T, Tanaka M, Nishimoto T. When overexpressed, a novel centrosomal protein, RanBPM, causes ectopic microtubule nucleation similar to gamma-tubulin. J Cell Biol. 1998;143:1041–1052. doi: 10.1083/jcb.143.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishitani H, Hirose E, Uchimura Y, Nakamura M, Umeda M, Nishii K, Mori N, Nishimoto T. Full-sized RanBPM cDNA encodes a protein possessing a long stretch of proline and glutamine within the N-terminal region, comprising a large protein complex. Gene. 2001;272:25–33. doi: 10.1016/s0378-1119(01)00553-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Li Z, Messing EM, Wu G. Activation of Ras/Erk pathway by a novel MET-interacting protein RanBPM. J Biol Chem. 2002;277:36216–36222. doi: 10.1074/jbc.M205111200. [DOI] [PubMed] [Google Scholar]
- 40.Hollenbeck P. Cytoskeleton: Microtubules get the signal. Curr Biol. 2001;11:R820–R823. doi: 10.1016/s0960-9822(01)00494-8. [DOI] [PubMed] [Google Scholar]
- 41.Blocker A, Gounon P, Larquet E, Niebuhr K, Cabiaux V, Parsot C, Sansonetti P. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J Cell Biol. 1999;147:683–693. doi: 10.1083/jcb.147.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]


