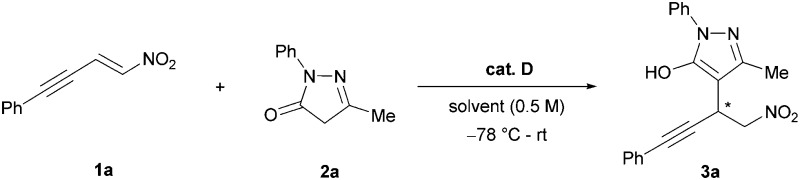
Table 1. Optimization of the asymmetric Michael addition a .
| ||||||
| Entry | Solvent | Cat. load (mol%) | T (°C) | t (h) | Yield b (%) | ee c (%) |
| 1 | CH2Cl2 | 5 | rt | 12 | 92 | 75 |
| 2 | Toluene | 5 | rt | 12 | 90 | 73 |
| 3 | CHCl3 | 5 | rt | 12 | 86 | 61 |
| 4 | Et2O | 5 | rt | 12 | 88 | 63 |
| 5 | CH2Cl2 | 5 | 0 | 0.5 | 93 | 89 |
| 6 | Toluene | 5 | 0 | 6 | 79 | 83 |
| 7 | CH2Cl2 | 5 | –20 | 2 | 93 | 92 |
| 8 | CH2Cl2 | 5 | –40 | 6 | 92 | 92 |
| 9 | CH2Cl2 | 5 | –78 | 6 | 92 | 92 |
| 10 | CH2Cl2 | 7 | –20 | 2 | 93 | 91 |
| 11 | CH2Cl2 | 3 | –20 | 2 | 93 | 92 |
| 12 | CH2Cl2 | 1 | –20 | 6 | 92 | 92 |
aReaction conditions: 0.28 mmol (1.1 equiv.) of 1a, 0.25 mmol (1.0 equiv.) of 2a, 1–7 mol% of catalyst D, 0.5 mL solvent.
bYield of isolated 3a after flash chromatography.
cThe enantiomeric excess was determined via the O-acetylated derivative 3a′ by HPLC analysis on a chiral stationary phase.
