Abstract
AIM: To study the difference in gene expression between solitary large hepatocellular carcinoma (SLHCC) and nodular hepatocellular carcinoma (NHCC).
METHODS: Polymerase chain reaction (PCR) products of 8464 human genes were spotted on a chip in array. DNAs were then fixed on a glass plate. Total RNA was isolated from freshly excised human SLHCC (n = 7) and NHCC (n = 15) tissues, and was reversely transcribed to cDNAs with the incorporation of fluorescent dUTP for preparation of hybridization probes. The mixed probes were then hybridized to the cDNA microarray. After highly stringent washing, cDNA microarray was scanned for the fluorescent signals to display the difference between the two kinds of HCC. In addition, the expression of RhoC and protocadherin LKC was also detected with the reverse transcriptase polymerase chain reaction (RT-PCR) method.
RESULTS: Among the 8464 human genes, 668 (7.89%) genes were expressed differentially at the mRNA levels between SLHCC and NHCC. Three hundred and fifty five (4.19%) genes, including protocadherin LKC, were up-regulated, whereas 313 (3.70%) genes, including RhoC, were down-regulated. The mRNA expression levels of RhoC and protocadherin LKC were confirmed by RT-PCR. Analysis of differentially expressed genes confirmed that our molecular data obtained by cDNA microarray were consistent with the published biochemical and clinical observations of SLHCC and NHCC.
CONCLUSION: cDNA microarray is an effective technique in screening the difference in gene expression between SLHCC and NHCC. Many of these differentially expressed genes are involved in the invasion and metastasis of HCC. Further analysis of these genes will help to understand the different molecular mechanisms of SLHCC and NHCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) ranks one of the most common malignancies in the world. Although its morbidity and mortality have decreased recently in patients with surgically treated HCC, the long-term prognosis remains unsatisfactory because of the high recurrence and metastasis rate. It has been generally accepted that the invasive and metastatic potentials of HCC are mostly attributed to the individual clinical pathological and molecular biological characteristics. The diversity of biological characteristics determines the different invasive and metastatic potentials of HCC[1]. In our institute, HCC was phenotypically divided into solitary large hepatocellular carcinoma (SLHCC, diameter > 5 cm, and one node), nodular hepatocellular carcinoma (NHCC, node number ≥ 2) and small hepatocellular carcinoma (SHCC, diameter ≤ 5 cm). Our clinical observation implied different invasive and metastatic abilities between SLHCC and NHCC. To understand the mechanism of different invasive and metastatic potentials between SLHCC and NHCC, the changes in gene expression between SLHCC and NHCC need to be investigated.
Our previous investigations focused on the differentially expressed genes of integrin, matrix metalloproteinases-2 (MMP-2), vascular endothelial growth factor (VEGF), phosphatase and tensin homologue deleted on chromosome ten (PETN), endoglin (CD105), survivin, which involved in invasion and metastasis, between SLHCC and NHCC[2]. Given the complex molecular mechanisms of invasion and metastasis, however, it is not clear that expression of these specific genes alone can explain the diversity of molecular biological characteristics between SLHCC and NHCC. Therefore, a broad evaluation of difference in gene expression between SLHCC and NHCC is necessary.
cDNA microarray represents an important new tool to analyze human gene expression profiles. The technology enables investigators to measure the expression of several thousand mRNAs simultaneously in a biological specimen. It is technically possible to monitor almost the entire transcriptosome, the collection of all mRNAs presented in a tumor specimen. cDNA microarray also enables us to study global gene profiles from various samples, thereby to speed up the identification of differentially expressed genes and the construction of different expression profiles. cDNA microarray analysis has become an increasingly popular tool to investigate the function of genes that are responsible for the phenotypes of diseases, to provide potential targets for treatment or prevention[3-5].
Our study was designed to delineate the different expression gene profiles between SLHCC and NHCC from 22 patients to evaluate the difference in gene expression within the realm of 8464 human genes for understanding the basement of the diversity of molecular biological characteristics between SLHCC and NHCC.
MATERIALS AND METHODS
Tissue specimens
The Ethics Committee of the Central South University approved the study protocol. Fresh surgical HCC was obtained from 22 (20 males and 2 females) patients, including 7 cases of SLHCC and 15 cases of NHCC with primary hepatocellular carcinoma who underwent hepatectomy at Xiangya Hospital of Central South University (CSU). The specimens were immediately freshly frozen in liquid nitrogen and stored at -80 °C for RNA isolation. The median age of the patients was 52 year (range, 27-73 years). All specimens obtained from surgery resection were confirmed by pathological examination.
RNA isolation
Total RNA was extracted from frozen tissue specimens (30-100 mg) using TRIZOL (GIBCO BRL, Gaithersburg, USA) reagent according to the instructions provided by the manufacturer. The quality was checked on 10 g/L agarose gels and the concentration was measured using an ultraviolet spectrophotometer (Biochrom Ltd, Cambridge, England). The uv wavelength was adjusted at 260 nm.
Construction of microarray
Microarray sequences included 8464 full-length and partial complementary DNAs representing known, novel, and control genes were provided by United Gene Holdings, Ltd, Shanghai, China. The cDNA inserts were amplified by PCR using universal primers to plasmid vector sequences and then purified as described previously[6]. The PCR products were then spotted onto silylated slides (CEL Associates, Houston, TX) using a Cartesian PixSys7500 motion control robot (Cartesian Technologies, Irvine, CA) fitted with Chipmaker micro-spotting technology (TeleChem International, Sunnyvale, CA). After spotting, the slide was exposed to ultraviolet light (65 mj/cm) and processed at room temperature by soaking in 2 g/L sodium dodecyl sulfate (SDS) for 10 min, distilled H2O for 10 min, and 2 g/L sodium borohydride (NaBH4) for 10 min. The slide was dried again and ready for use.
Probes of expression profile preparation
Individual RNA specimens from the same group of HCC were mixed equally to obtain 60 µg total RNA. Fluorescent cDNA probes were prepared through reverse transcription and then purified. RNA samples extracted from NHCC were labeled with Cy3-dUTP and those from SLHCC with Cy5-dUTP. The two color probes were then mixed, precipitated with ethanol and dissolved in 20 µL of hybridization solution containing 5 × SSC (0.75 moL NaCl and 0.075 moL sodium citrate), 4 g/L SDS, 500 g/L formamide and 5 × Denhardt’s solution (1 g/L Ficoll, 1 g/L polyvinylpyrrolidone and 1 g/L bovine serum albumin).
Hybridization and washing
Microarrays was prehybridized with hybridization solution containing 5 g/L denatured salmon sperm DNA at 42 °C for 6 h. Fluorescent probe mixtures were denatured at 95 °C for 5 min, and the denatured probe mixtures were applied onto the prehybridized chip under a cover glass. Chips was hybridized at 42 °C for 18 h. The hybridized chips was then washed at 60 °C for 10 min each in solutions of 2 × SSC and 2 g/L SDS, 0.1 × SSC and 2 g/L SDS, and 0.1 × SSC, then dried at room temperature.
Detection and analysis
The chip was scanned with Scan Array 4000 standard biochip scanning system (Packard Biochip Technologies, Virginia, USA). ImaGene 3.0 software (Bio Discovery, Los Angeles, USA) was used to analyze the intensity of spots. The intensities of each spot at the two wavelengths represented the quantity of Cy3-dUTP and Cy5-dUTP, respectively, hybridized to each spot. The ratio of Cy5 to Cy3 was computed for each location. Overall intensities were normalized with a correction coefficient obtained using the ratios of the housekeeping genes. Genes were identified as differentially expressed if the absolute value of the natural logarithm of the ratio was > 0.69. To minimize artifacts arising from low expression values, the genes with raw intensity values for Cy3 and Cy5 > 800 counts were chosen for differential analysis.
RT-PCR
Total RNA (2 µg) was reversely transcribed in a final 25 µL reaction volume at 37 °C for 1 h by using 200 U M-MULV reverse transcriptase (Promega, Madison, USA). PCR amplification was performed in a final volume of 50 µL containing 5 µL of first strand cDNA solution, 2 U of Taq polymerase (Sangon, Shanghai, China), 5 µL of 10 × PCR reaction buffer, 10 µmol/L of dNTP (Sangon, Shanghai, China) and 10 pmoL of each 3’ and 5’ sequence specific oligonucleotide primer (Sangon, Shanghai, China) for RhoC, protocadherin LKC and β2-microglobulin gene (positive control). The amplification was performed on a DNA thermal cycler (Perkin Elmer, Shelton, USA). The primer sequences and the annealing temperature are shown in Table 1. PCR products were electrophoresed on 17 g/L agarose gels. The bands representing amplified products were visualized using ethidium bromide during the exposure to a UV transilluminator. The density of the bands on the gel was quantified by densitometry analysis. The expression of the genes was presented by the relative intensity of the PCR product bands from target sequences to that from the β2-microglobulin gene[7].
Table 1.
Primer sequences and annealing temperature for PCR
| Gene | Primer sequences | Product size | Annealing temperature | |
| RhoC | Up | 5’-TCCTCATCGTCTTCAGCAAG-3’ | 183 bp | 56 °C |
| Down | 5’-CTGCAATCCGAAAGAAGCTG-3’ | |||
| protocadherin | Up | 5’-GAAGCTTCAAGCTATGAAAA-3 | 256 bp | 52 °C |
| LKC | Down | 5’-CCTTGATTTCCTGACTGTTC-3’ | ||
| β2-MG | Up | 5’-ACCCCCACTGAAAAAGATGA-3’ | 120 bp | 56 °C |
| Down | 5’-ATCTTCAAACCTCCATGATG-3’ |
Statistical analysis
Statistical analysis was performed using the SPSS (version 11.0, Chicago, IL). The nonparametric Mann-Whitney test was performed to evaluate the differences in the expression of RhoC and protocadherin LKC between the groups. P < 0.05 was considered statistically significant.
RESULTS
Quality and examination of total RNA
Ethidium bromide-stained total RNA agarose gel of SLHCC and NHCC was performed (Figure 1). None of our analyzed samples had detectable RNA degradation present at the leading edge of the gel, which is critical for reproducible gene expression data. The productive rate of RNA from SLHCC and NHCC was 0.42-1.64 mg/g and 0.34-1.85 mg/g, respectively. The rate of A260/A280 was 1.81-2.05. These results showed the high quality and purity of total RNA obtained.
Figure 1.
Ethidium bromide-stained 1% agarose gel of four RNA samples. There were sharp ribosomal bands and mini-mal degradation that was critical for expression analysis. Lanes 1 and 2 represent total RNA derived from SLHCC. Lanes 3 and 4 represent total RNA from NHCC.
Identification of genes differentially expressed between SLHCC and NHCC
By applying the threshold value of 2 (up-regulation) and 0.5 (down-regulation) for intensity ratio of SLHCC vs NHCC, 668 genes (7.89%) were selected as differentially expressed genes, including 355 up-regulated (4.19%) and 313 down-regulated (3.69%) genes (Figure 2). Consistent with the precision of the assay, scatter plots comparing SLHCC with NHCC showed a wide distribution of the ratio in which red or green spots represented differently expressed genes, while yellow spots were related to similarly expressed genes (Figure 3).
Figure 2.

cDNA microarray slide hybridized to SLHCC and NHCC shown before image analysis. The slide shown was spotted with 8464 genes, hybridized to fluorescence-labeled cDNA, and exposed to scanArray 4000 for image capture. Red spots represent up-regulated genes, green spots represent down-regulated genes in SLHCC compared with NHCC. Yellow spots are related to genes of which gene expression was similar.
Figure 3.

Scatter plot with ImaGene 3.0 software after hybrid-ization of SLHCC (Cy5)/NHCC (Cy3) (8464 elements). Red spots represent similar expression genes. Yellow spots repre-sent different expression genes.
The result revealed that the activated and repressed genes represented a different subset of cellular genes with biochemical activities consistent with the physiology and pathology between SLHCC and NHCC. Collectively, the genes provided a quantitative view of the changes in gene expression that occurred at the level of the human genome between SLHCC and NHCC. Based on the function discrepancy of encoding proteins, these variably expressed genes were mainly divided into 15 classes (Table 2).
Table 2.
Numbers of variably expressed genes in SLHCC and NHCC
| Class | No. of up-regulated genes | No. of down-regulated genes |
| Oncogenes and antioncogenes | 6 | 5 |
| Genes encoding proteins related to ionic channel and protein transportation | 7 | 7 |
| Genes encoding cellular cycle modulator | 3 | 9 |
| Genes associated with cellular stress | 1 | 1 |
| Genes associated with cellular skeleton and movement | 5 | 7 |
| Genes related to cellular apoptosis | 2 | 2 |
| Gene encoding proteins regulating synthesis, repair, and rearrangement of DNA | 0 | 4 |
| Genes encoding transcription factors and proteins binding with DNA | 17 | 6 |
| Cytokine receptor gene | 4 | 3 |
| Immunological protein gene | 18 | 17 |
| Genes associated with cellular metabolism | 32 | 32 |
| Genes encoding signal transductional factor | 39 | 31 |
| Genes encoding factors that regulated protein translation | 11 | 37 |
| Genes encoding proteins that were consistent with body growth and development | 7 | 8 |
| Other | 203 | 144 |
Detection mRNA expression of RhoC and protocadherin LKC in SLHCC and NHCC by RT-PCR
Expression of RhoC and protocadherin LKC mRNA was detected in all SLHCC and NHCC. SLHCC tissues revealed significantly higher levels of protocadherin LKC mRNA than NHCC (P = 0.026), and showed significantly lower levels of RhoC mRNA than NHCC (P = 0.031) (Figure 4). The results were consistent with the results of cDNA microarray.
Figure 4.
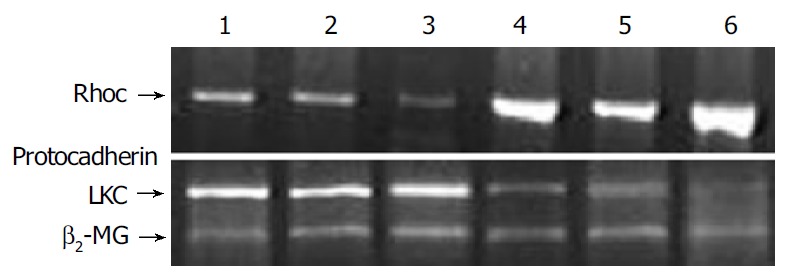
Expression of RhoC and protocadherin LKC mRNA in SLHCC (lanes 1-3) and NHCC (lanes 4-6) SLHCC tissues revealed significantly higher levels of protocadherin LKC mRNA than NHCC and showed significantly lower levels of RhoC mRNA than NHCC.
DISCUSSION
Traditional viewpoint insists that the classification and prognosis of HCC are determined by the size of HCC, large HCC is always considered as terminal HCC and unexcisable HCC. However, UICC has changed this opinion in the 6th TNM classification for HCC. The number of nodes, but not the size of HCC, is the crucial factor for classification and prognosis of HCC[8]. It is generally accepted that different HCCs have individual clinical pathological and molecular biological characteristics, which determined the different invasive and metastatic potentials of HCC[1]. Our clinical observation implied different invasive and metastatic abilities between SLHCC and NHCC. As the genomewide expression profile of tumor is a representation of the biology of the tumor, and the diversity in gene expression profile reflects molecular biological diversity, thus, the further investigation of different gene expression profiles between SLHCC and NHCC is anticipated.
In this study, systematic investigation of gene expression patterns between SLHCC and NHCC was performed by using cDNA microarray to understand the mechanism of the diversity of molecular biological characteristics of HCC. A total of 668 genes (7.89%) showed significant change in their expression level between SLHCC and NHCC. It is not surprising that some differentially expressed genes were associated with the progression of HCC and most of them were related to the invasion and metastasis of tumor. These findings lend support to the presence of various molecular characteristics between SLHCC and NHCC. The gene expression profiles also provided a quantitative view of the changes in gene expression that occurred at the level of HCC genome between SLHCC and NHCC. These genes include matrix-degraded gene, cellular skeleton and motion gene, cellular signal and transferring gene, apoptosis-associated gene and so on. Some genes which were propitious to invasion and metastasis were up-regulated and made against these were down-regulated. We focused on matrix-degraded genes, cellular skeleton and motion genes, which were closely related to invasion and metastasis, and selected some of them for further study.
Both cell-cell interaction and cell-stroma interaction play an important role during the invasion and metastasis. Connections through cell-adhesion molecules, integrins, and cadherins stabilize tissue integrity, whereas loss or alteration of these cell surface proteins is associated with increased metastatic potential. The results of our study indicated that cathepsinL (CTSL), integrinα6, (ITGA6) were down-regulated in SLHCC compared with NHCC. We also found that protocadherin LKC gene and H-cadherin gene were overexpressed in SLHCC vs NHCC. CTSL could promote migration of HCC cells through degrading ECM[9,10]. ITGA6 could promote invasion and metastasis of HCC cells through activing MMPS[11]. Loss of H-cadherin expression in invasive cutaneous squamous cell carcinoma suggested it was a potential invasive and metastatic suppressive gene[12]. Protocadherins are a major subfamily of the cadherin superfamily. This gene is predominantly expressed in liver, kidney and colon tissues, thus designating protocadherin LKC. The expression of protocadherin LKC is markedly reduced in cancers arising from these tissues at both transcriptional and protein levels. When protocadherin LKC was introduced into colon cancer cell line HCT116, which does not express this gene, a significant inhibition of cell proliferation could be observed. Recently, researcher identified the status of protocadherin LKC as an invasion and metastasis suppressor or gene which could increase the coagulation of tumor cells[13-16]. Our study showed that protocadherin LKC mRNA was down-regulated in NHCC, suggesting a more disperse degree of NHCC cells compared with SLHCC cells.
We also found that many genes associated with cellular skeleton and movement were differentially expressed between SLHCC and NHCC, for instance, RAB34 and RhoC. We focussed on RhoC because it was recently reported to play a crucial role in tumorigenesis. RhoC was found overexpressed in pancreatic ductal adenocarcinomas[7], and its up-regulation was associated with tumor progression in ovarian carcinomas[17]. Our previous study also showed a significant higher level of RhoC in extrahepatic metastasis of HCC compared with intrahepatic HCC and overexpression of RhoC gene was correlated with vein invasion, number of tumor nodes and extrahepatic metastasis[18]. The expression level of RhoC could reflect the mobility of HCC cells[19-22]. The results of our study that RhoC was down-regulated in SLHCC compared with NHCC suggested that SLHCC cells had a lower invasive and metastatic potential than NHCC.
Apoptosis is thought to play an important role in the regulation of proliferation of HCC cells. Apoptosis is modulated by a number of gene products which act through protein-protein interactions either as inducers or inhibitors. In our study, the genes of DAP, PHLDA, ATF2, p21waf1/cip1, and their products which could induce cellular apoptosis[22-26], were obviously up-regulated in SLHCC compared with NHCC, whereas survivin, which inhibited cellular apoptosis[27-31] was less in SLHCC than in NHCC. These data suggested that one of the mechanisms of SLHCC, which possesses a less invasive and metastatic potential than NHCC, might be a facility in apoptosis of HCC cells. Our study also showed many genes encoding proteins that were consistent with body growth and development were down-regulated in SLHCC compared with NHCC, for instance, SNRPN, NTRK3 and so on[32,33]. These genes express usually in embryonic tissues and reflect the level of cell differentiation. htese result suggested SLHCC cells has a better differentiation than NHCC cells.
In conclusion, the diversity of molecular biological characters between SLHCC and NHCC can be determined by differentially expressed gene profiles. SLHCC shares a better gene phenotype than NHCC, which is consistent with our previous clinical study.
ACKNOWLEDGMENTS
We are grateful to Zhi-Li Yang, Wei-Qun Lu, Fa-Qing Tang, Jian-Qing Yang and He-Li Liu for their technical assistance.
Footnotes
Supported by National Key Technologies R and D Program of China during the 10th Five-year plan period, No. 2001BA703B04 and National Natural Science Foundation of China, No. 30371595 and Hunan Province Developing Planning Committee, No. 2001-907
Edited by Kumar M and Wang XL Proofread by Xu FM
References
- 1.Okuda K. Hepatocellular carcinoma: clinicopathological aspects. J Gastroenterol Hepatol. 1997;12:S314–S318. doi: 10.1111/j.1440-1746.1997.tb00515.x. [DOI] [PubMed] [Google Scholar]
- 2.Huang G, Yang L, Yang J, Liu H, Yang Z. [Correlation between epidermal growth factor and overexpression of vascular endothelial growth factor in hepatocellular carcinoma] Zhonghua Zhongliu Zazhi. 2002;24:564–566. [PubMed] [Google Scholar]
- 3.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 4.Cherepinsky V, Feng J, Rejali M, Mishra B. Shrinkage-based similarity metric for cluster analysis of microarray data. Proc Natl Acad Sci USA. 2003;100:9668–9673. doi: 10.1073/pnas.1633770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King HC, Sinha AA. Gene expression profile analysis by DNA microarrays: promise and pitfalls. JAMA. 2001;286:2280–2288. doi: 10.1001/jama.286.18.2280. [DOI] [PubMed] [Google Scholar]
- 6.Schena M. Genome analysis with gene expression microarrays. Bioessays. 1996;18:427–431. doi: 10.1002/bies.950180513. [DOI] [PubMed] [Google Scholar]
- 7.Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, Narumiya S, Hiai H, Fukumoto M. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77:147–152. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST. Evaluation of the new AJCC/UICC staging system for hepatocellular carcinoma after hepatic resection in Chinese patients. Surg Oncol Clin N Am. 2003;12:35–50, viii. doi: 10.1016/s1055-3207(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 9.Atkins KB, Troen BR. Phorbol ester stimulated cathepsin L expression in U937 cells. Cell Growth Differ. 1995;6:713–718. [PubMed] [Google Scholar]
- 10.Niedergethmann M, Hildenbrand R, Wolf G, Verbeke CS, Richter A, Post S. Angiogenesis and cathepsin expression are prognostic factors in pancreatic adenocarcinoma after curative resection. Int J Pancreatol. 2000;28:31–39. doi: 10.1385/IJGC:28:1:31. [DOI] [PubMed] [Google Scholar]
- 11.Hourihan RN, O'Sullivan GC, Morgan JG. Transcriptional gene expression profiles of oesophageal adenocarcinoma and normal oesophageal tissues. Anticancer Res. 2003;23:161–165. [PubMed] [Google Scholar]
- 12.Takeuchi T, Liang SB, Matsuyoshi N, Zhou S, Miyachi Y, Sonobe H, Ohtsuki Y. Loss of T-cadherin (CDH13, H-cadherin) expression in cutaneous squamous cell carcinoma. Lab Invest. 2002;82:1023–1029. doi: 10.1097/01.lab.0000025391.35798.f1. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki N, Takahashi N, Kojima S, Masuho Y, Koga H. Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation. Carcinogenesis. 2002;23:1139–1148. doi: 10.1093/carcin/23.7.1139. [DOI] [PubMed] [Google Scholar]
- 14.Chen MW, Vacherot F, De La Taille A, Gil-Diez-De-Medina S, Shen R, Friedman RA, Burchardt M, Chopin DK, Buttyan R. The emergence of protocadherin-PC expression during the acquisition of apoptosis-resistance by prostate cancer cells. Oncogene. 2002;21:7861–7871. doi: 10.1038/sj.onc.1205991. [DOI] [PubMed] [Google Scholar]
- 15.Wolverton T, Lalande M. Identification and characterization of three members of a novel subclass of protocadherins. Genomics. 2001;76:66–72. doi: 10.1006/geno.2001.6592. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki ST. Recent progress in protocadherin research. Exp Cell Res. 2000;261:13–18. doi: 10.1006/excr.2000.5039. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–870. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Yang LY, Yang ZL, Huang GW, Lu WQ. Expression and significance of RhoC gene in hepatocellular carcinoma. World J Gastroenterol. 2003;9:1950–1953. doi: 10.3748/wjg.v9.i9.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donald CD, Cooper CR, Harris-Hooker S, Emmett N, Scanlon M, Cooke DB. Cytoskeletal organization and cell motility correlates with metastatic potential and state of differentiation in prostate cancer. Cell Mol Biol (Noisy-le-grand) 2001;47:1033–1038. [PubMed] [Google Scholar]
- 20.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 21.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 22.Zondag GC, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–782. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto H, Nagao M, Ogawa S, Kanehiro H, Hisanaga M, Ko S, Ikeda N, Fujii H, Koyama F, Mukogawa T, et al. Prognostic significance of death-associated protein-kinase expression in hepatocellular carcinomas. Anticancer Res. 2003;23:1333–1341. [PubMed] [Google Scholar]
- 24.Neef R, Kuske MA, Pröls E, Johnson JP. Identification of the human PHLDA1/TDAG51 gene: down-regulation in metastatic melanoma contributes to apoptosis resistance and growth deregulation. Cancer Res. 2002;62:5920–5929. [PubMed] [Google Scholar]
- 25.Bhoumik A, Huang TG, Ivanov V, Gangi L, Qiao RF, Woo SL, Chen SH, Ronai Z. An ATF2-derived peptide sensitizes melanomas to apoptosis and inhibits their growth and metastasis. J Clin Invest. 2002;110:643–650. doi: 10.1172/JCI16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 27.Krysan K, Merchant FH, Zhu L, Dohadwala M, Luo J, Lin Y, Heuze-Vourc'h N, Põld M, Seligson D, Chia D, et al. COX-2-dependent stabilization of survivin in non-small cell lung cancer. FASEB J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y, Kuroiwa T, Nakagawa T, Kajimoto Y, Dohi T, Azuma H, Tsuji M, Kami K, Miyatake S. Transcriptional expression of survivin and its splice variants in brain tumors in humans. J Neurosurg. 2003;99:738–745. doi: 10.3171/jns.2003.99.4.0738. [DOI] [PubMed] [Google Scholar]
- 29.Lo Muzio L, Pannone G, Leonardi R, Staibano S, Mignogna MD, De Rosa G, Kudo Y, Takata T, Altieri DC. Survivin, a potential early predictor of tumor progression in the oral mucosa. J Dent Res. 2003;82:923–928. doi: 10.1177/154405910308201115. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. [PubMed] [Google Scholar]
- 31.Grabowski P, Scherubl H. Survivin--an anti-apoptosis protein. Med Sci Monit. 2003;9:LE25. [PubMed] [Google Scholar]
- 32.Bussey KJ, Lawce HJ, Himoe E, Shu XO, Heerema NA, Perlman EJ, Olson SB, Magenis RE. SNRPN methylation patterns in germ cell tumors as a reflection of primordial germ cell development. Genes Chromosomes Cancer. 2001;32:342–352. doi: 10.1002/gcc.1199. [DOI] [PubMed] [Google Scholar]
- 33.Hisaoka M, Sheng WQ, Tanaka A, Hashimoto H. Gene expression of TrkC (NTRK3) in human soft tissue tumours. J Pathol. 2002;197:661–667. doi: 10.1002/path.1138. [DOI] [PubMed] [Google Scholar]


