Figure 4.
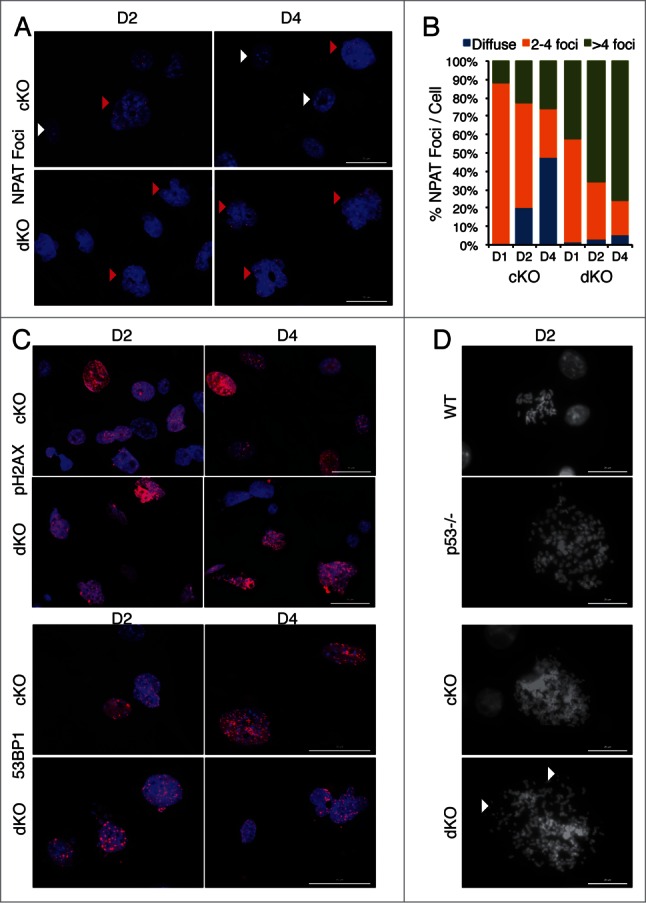
Removal of p53 in Hinfp null cells alters histone locus body (HLB) patterns and induces genomic instability. (A) IF microscopy shows the distribution of HLB by staining for the marker protein NPAT (red), an integral component of HLBs. cKO MEFs show an increase in the fraction of cells with multiple NPAT foci (red arrowheads) or diffused NPAT staining (white arrowheads) after Hinfp depletion. dKO MEFs show a further increase in cells with multiple NPAT foci. Nuclei were co-stained with DAPI (blue). Scale Bar 50 µm. (B) Quantitation of NPAT staining patterns in cKO and dKO MEFs at d1, d2 and d4 after removal of Hinfp. Nuclei were counted from 2 biological replicates (200 each) per sample for each time point. (C) cKO and dKO MEFs were analyzed for factors associated with double-strand DNA damage using immunofluorescence microscopy. (Upper panels) γ-H2Ax S-139 (red) and Scale Bar 50 µm. (Lower panels) 53BP1 (red) Scale Bar 50 µm. Nuclei were co-stained with DAPI (blue). Both cKO and dKO MEFs show cells with focal staining for both γ-H2Ax and 53BP1 that is even higher in dKO cells. (D) WT, p53−/−, cKO and dKO MEFs were mitotically arrested at D2 using Colcemid (100 ng/ml). The mitoses were analyzed by fluorescence microscopy. The DNA was stained with DAPI. cKO cells have an increased chromosome complement compared to WT, and there is increased appearance of chromosomal fragility (white arrowheads) in cells lacking both p53 and Hinfp. Scale Bar 20 µm.
