Figure 1.
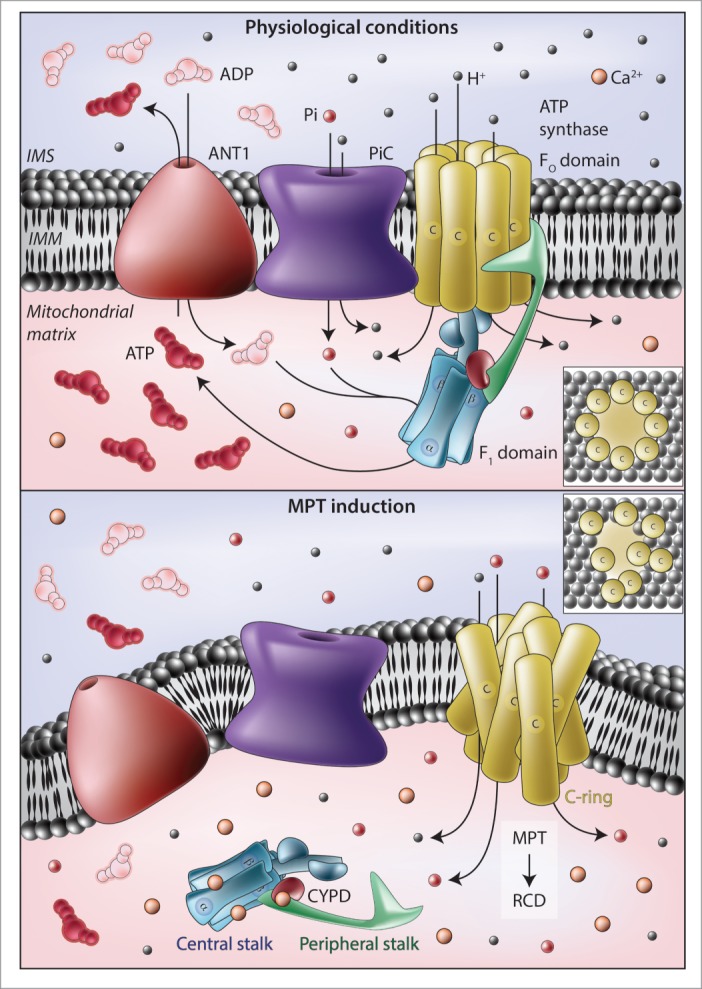
Implication of the ATP synthasome in the mitochondrial permeability transition. (A) In physiological conditions, the F1FO ATP synthase harnesses the electrochemical gradient established across the inner mitochondrial membrane (IMM) by respiratory chain complexes to produce ATP. The substrates of this reaction, i.e., ADP and inorganic phosphate (Pi), are provided by various members of the solute carrier (SLC) protein family, including SLC25A4, best known as adenine nucleotide translocase 1 (ANT1) and SLC25A3, best known as mitochondrial phosphate carrier (PiC). In particular, ANT1 exports ATP from the mitochondrial matrix in exchange of ADP (both of which are transported along their concentration gradient), whereas PiC operates as an H+-driven Pi/H+ symporter. (B) In response to oxidative stress or cytosolic Ca2+ overload, Ca2+ ions accumulate in the mitochondrial matrix and bind to the F1 domain of the F1FO ATP synthase, an activity that may be regulated by peptidylprolyl isomerase F (PPIF, best known as cyclophilin D, CYPD). In these conditions, F1 domains appear to dissociate from their IMM-embedded interacting partners (FO domains), allowing c-rings to structurally rearrange and form relatively unselective pores that initiate the mitochondrial permeability transition (MPT). Both ANT1 and PiC have been shown to influence the propensity of cells to undergo MPT-driven regulated cell death (RCD), but the precise molecular mechanisms remain elusive. IMS, mitochondrial intermembrane space.
