Table 2. Substrate scope a .
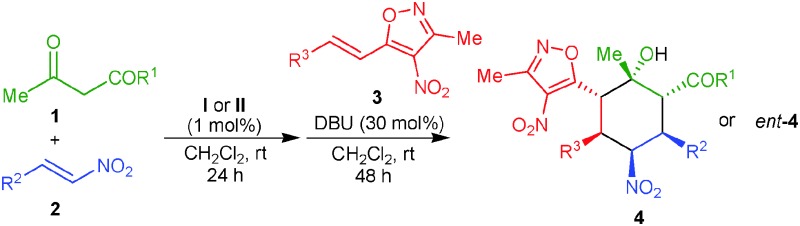
| |||||
| 4/ent-4 | R1 | R2 | R3 | Yield b (%) | ee c (%) |
| 4a | OEt | Ph | Ph | 61 | 98 |
| 4b | OEt | 4-FC6H4 | Ph | 64 | 99 |
| 4c | OEt | 4-ClC6H4 | Ph | 55 | 99 |
| 4d | OEt | 4-MeC6H4 | Ph | 63 | 93 |
| 4e | OEt | 4-MeOC6H4 | Ph | 67 | 97 |
| 4f | OEt | 2-Thienyl | Ph | 61 | 91 |
| 4g | OEt | Ph | 4-FC6H4 | 60 | 98 |
| 4h | OEt | Ph | 4-ClC6H4 | 61 | 97 |
| 4i | OEt | Ph | 3-ClC6H4 | 69 | 97 |
| 4j | OEt | Ph | 4-MeC6H4 | 73 | 99 |
| 4k | OEt | Ph | 2-MeC6H4 | 49 | 95 |
| 4l | OEt | Ph | 4-MeOC6H4 | 39 | 96 |
| 4m | OEt | Ph | 2-Thienyl | 50 | 97 |
| 4n | OMe | Ph | Ph | 58 | 97 |
| 4o | Me | Ph | Ph | 50 | 96 |
| ent-4a | OEt | Ph | Ph | 69 | 96 |
| ent-4b | OEt | 4-FC6H4 | Ph | 63 | 97 |
| ent-4c | OEt | 4-ClC6H4 | Ph | 51 | 95 |
| ent-4d | OEt | 4-MeC6H4 | Ph | 64 | 98 |
| ent-4e | OEt | 4-MeOC6H4 | Ph | 66 | 95 |
| ent-4f | OEt | 2-Thienyl | Ph | 59 | 96 |
| ent-4h | OEt | Ph | 4-ClC6H4 | 60 | 97 |
| ent-4k | OEt | Ph | 2-MeC6H4 | 50 | 96 |
aReaction conditions: 0.5 mmol of 1, 0.5 mmol of 2, 1 mol% of I (entry 1–17) or II, 1.0 mmol of 3 and 30 mol% of DBU (0.1 M in CH2Cl2).
bYield of isolated product after column chromatography.
cEnantiomeric excess of the major diastereomer determined by HPLC analysis on a chiral stationary phase.
