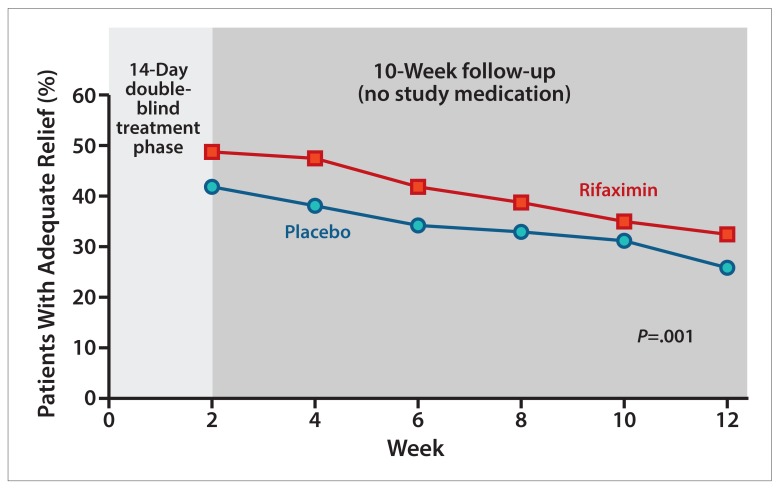
Figure 6.
Patients with adequate relief of global IBS symptoms in TARGET 1 and TARGET 2 during the 10-week follow-up period after cessation of treatment with rifaximin or placebo. IBS, irritable bowel syndrome.
Adapted from Pimentel M et al. N Engl J Med. 2011;364(1):22-32.20