Abstract
Calorie restriction (CR) without malnutrition extends life span in several animal models. It has been proposed that a decrease in the amount of polyunsaturated fatty acids (PUFAs), and especially n-3 fatty acids, in membrane phospholipids may contribute to life span extension with CR. Phospholipid PUFAs are sensitive to dietary fatty acid composition, and thus, the purpose of this study was to determine the influence of dietary lipids on life span in CR mice. C57BL/6J mice were assigned to four groups (a 5% CR control group and three 40% CR groups) and fed diets with soybean oil (high in n-6 PUFAs), fish oil (high in n-3 PUFAs), or lard (high in saturated and monounsaturated fatty acids) as the primary lipid source. Life span was increased (p < .05) in all CR groups compared to the Control mice. Life span was also increased (p < .05) in the CR lard mice compared to animals consuming either the CR fish or soybean oil diets. These results indicate that dietary lipid composition can influence life span in mice on CR, and suggest that a diet containing a low proportion of PUFAs and high proportion of monounsaturated and saturated fats may maximize life span in animals maintained on CR.
Key Words: Calorie restriction, Life span, Aging, Dietary fat, Fatty acids
Calorie restriction (CR) without malnutrition extends life span and delays the onset of age-related diseases in a wide range of animal models (reviewed in (1)). However, its mechanisms of action are not fully understood. Consistent with the free radical theory of aging (2), it has been proposed that a decrease in reactive oxygen species (ROS) production and tissue oxidative damage is a major factor contributing to life span extension with CR (3, 4). Membrane phospholipids are a major site of oxidative damage and this has led to an offshoot of the free radical theory which suggests that life span is inversely related to the level of unsaturation of phospholipids (5, 6). It has been reported that CR induces a decrease in the amount of long chain polyunsaturated fatty acids (PUFAs) (7, 8), and thus, it has been suggested that CR increases life span by producing membranes that are resistant to oxidative damage (9). These theories that link mitochondrial phospholipid fatty acid composition to aging focus on lipid peroxidation as the mechanism through which fatty acids influence life span. However, mitochondrial phospholipid fatty acids could also influence aging by altering the activity of membrane proteins (10–14), membrane permeability (15–17), ROS production (18–20), or other membrane-linked processes. Therefore, it is possible that phospholipid fatty acids may influence the actions of CR by altering susceptibility to oxidative damage or by mechanisms distinct from oxidative stress.
Since mitochondrial phospholipid fatty acid composition in CR mice can be altered by dietary fats (18, 21), it follows that changes in dietary fatty acid composition could influence life span in CR animals. Several studies have evaluated the potential effect of different dietary fat sources on life span in rodents, although these studies have yielded mixed results. Diets containing a high proportion of n-3 fatty acids have been reported to decrease life span in senescence-accelerated mice (22, 23) and diabetic rats (24). In contrast, diets with a high n-3/n-6 fatty acid ratio did not alter life span in wild-type Wistar rats (25) and diets containing fish oil (high in long chain n-3 fatty acids) increased life span in autoimmune lupus-prone mice (26, 27). However, these studies have largely been completed in short-lived rodent models allowed free choice consumption of food and there is little information about the influence of dietary fatty acids on life span of long-lived CR mice.
We have previously shown in CR mice that dietary fat source modulates, in a tissue-dependent manner, mitochondrial membrane composition, proton leak, H2O2 production, and electron transport chain enzyme activities (18, 21, 28). Additionally, we have shown that dietary fat source influences apoptotic signaling and mitochondrial ultrastructure in CR mice (29, 30). However, it is not known if alterations in dietary lipid composition have an impact on life span in CR mice. We hypothesized that life span will be increased in CR mice consuming a diet with a high proportion of monounsaturated and saturated fatty acids compared to CR animals consuming a diet with a high proportion of PUFAs. To test this hypothesis, life span and end-of-life pathology were measured in CR mice maintained at the same level of energy intake (8.6 kcal/d) but fed diets which differed in the primary dietary fat source (soybean oil, fish oil, or lard).
Methods
Animal Husbandry
Male C57BL/6J mice were purchased at 2 months of age from the Jackson Laboratory (Sacramento, CA) and fed a commercial rodent chow diet (Harlan Teklad #7012, Madison, WI). All mice were individually housed in polycarbonate cages placed within positive pressure, HEPA filtered units. The mice were housed at 22–24 °C and 40%–60% relative humidity with a 12-hour light/12-hour dark cycle. All animals had unlimited access to water throughout the study. All experimental procedures were approved by the University of California, Institutional Animal Care and Use Committee.
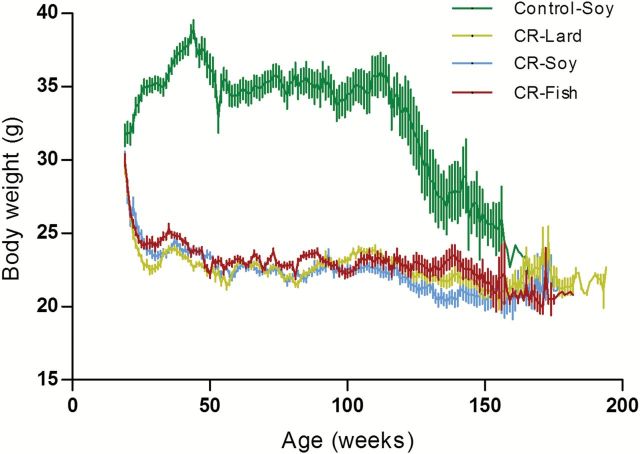
Food intake was measured on the mice from 3–4 months of age, and average ad libitum food intake was calculated for the entire group of mice. At 4 months of age, the animals were randomly assigned to four dietary groups of 40 mice each. All groups were fed modified AIN-93G semi-purified diets. The control group was fed 95% of the average ad libitum intake (13.6 kcal/d). This slight restriction prevented excessive weight gain in this group during the course of the study, and average body weight was approximately 33g for this group of mice over the course of the study. The three CR groups were fed 60% of ad libitum intake (8.6 kcal/d). The modified AIN-93G diets (% of total kcal) each contained 20.3% protein, 63.8% carbohydrate, and 15.9% fat. Soybean oil was the dietary fat in the control group (standard AIN-93G diet). The dietary fats for the CR groups were soybean oil (high in n-6 fatty acids, 55% linoleic acid, Super Store Industries, Lathrop, CA), lard (high in monounsaturated and saturated fatty acids, ConAgra Foods, Omaha, NE) and fish oil (high in n-3 PUFAs, 18% eicosapentaenoic acid, 12% docosahexaenoic acid, Jedwards International, Inc., Quincy, MA). To meet linoleic acid requirements, the fish oil diet contained 1% (w/w) soybean oil. A detailed composition of the diet has been previously published (18). At 6 months of age, all mice were switched to modified AIN-93M diets containing the same dietary lipids they consumed previously (Table 1). The amount of food offered to the animals was adjusted to make certain that daily energy intake (kcal/d) for each group remained the same after the diet switch. The purpose of the diet switch was to mitigate the amount of excess protein consumed by the mice, since the protein as a percentage of energy is 20.3% in the AIN-93G diet versus 14.8% in the AIN-93M diet. Thus, the AIN-93M diet provided protein at a level closer to the maintenance protein requirement for laboratory rodents (31). To meet linoleic acid requirements, soybean oil was added to both the fish oil and lard diets (Table 1). Each of the modified AIN-93M diets (% of total kcal) contained 14.8% protein, 75.7% carbohydrate, and 9.5% fat. The stock fish oil was stored frozen under nitrogen gas. All diets were prepared every 3–4 months. The fish oil diet was stored at −20 °C and the soybean oil and lard diets were stored at 4 °C until fed.
Table 1.
Dietary Composition
| Dietary Group (g/100g diet) | |||
|---|---|---|---|
| Ingredients | Control/CR Soy | CR Fish | CR Lard |
| Corn starch | 46.5 | 46.5 | 46.5 |
| Casein | 14.0 | 14.0 | 14.0 |
| Maltodextrin | 15.1 | 15.1 | 15.1 |
| Sucrose | 10.0 | 10.0 | 10.0 |
| Soybean oil* | 4.0 | 1.2 | 0.7 |
| Fish oil† | 0 | 2.8 | 0 |
| Lard‡ | 0 | 0 | 3.3 |
| Cellulose | 5.0 | 5.0 | 5.0 |
| Mineral mix #94049 | 3.5 | 3.5 | 3.5 |
| Vitamin mix #94047 | 1.0 | 1.0 | 1.0 |
| L-Cysteine | 0.18 | 0.18 | 0.18 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 |
| t-Butylhydroquinone | 0.0008 | 0.0016 | 0.0008 |
Notes: *Containing 16:0 (10.2%), 18:1 n-9 (21.2%), and 18:2 n-6 (55%) as the most abundant fatty acids.
†Containing 14:0 (7.5%), 16:0 (16.5%), 16:1 n-7 (9.6%), 18:1 n-9 (8.7%), 18:2 n-6 (1.3%), 20:5 n-3 (17.7%), and 22:6 n-3 (10.3%) as the most abundant fatty acids.
‡Containing 16:0 (23.7%), 18:0 (15%), 18:1 n-9 (39.2%), and 18:2 n-6 (15%) as the most abundant fatty acids.
The mice in all groups were fed daily in the afternoon, approximately 5–6 hours before the start of the dark cycle. Health checks were completed on the animals at least once per day and body weight was measured for each animal on a weekly basis. No other procedures were completed on the mice in this study. The mice were allowed to live out their natural life span, and animals were only euthanized if they were moribund. Mice were considered moribund if they exhibited a lack of response to an external stimulus, inability to reach food or water, or an ulcerated/bleeding tumor. Less than 10% of the mice were euthanized in the study, and these mice were spread among all four diet groups. Animals found dead in their cages or euthanized were placed in 10% formalin solution after opening their body cavities. The mice were then submitted to the UC Davis Comparative Pathology Laboratory for gross necropsy and histologic examination. Date of death was recorded and used to calculate life span.
The mice were housed in three individually filtered and ventilated racks. This room also contained one rack housing animals involved in separate experiments. Sentinel mice were housed on the same racks as the study animals and exposed to bedding from the study mice on a weekly basis. Sentinels were euthanized every 3 months for health screens, including aerobic cultures and serology (MHV, MPV, MVM, M. pul., TMEV [GDVII], Ectro, EDIM, MAD1, MAD2, LCM, Reo-3). All tests were negative for sentinels on the life span racks throughout the study. However, midway through the study a sentinel on the rack housing mice involved in separate experiments tested positive for MPV. All mice on the rack containing the sentinel were euthanized and tested for MPV. All the animals were negative. To make certain that the enclosed racks containing the life span mice were clean; mice were randomly selected from these racks, euthanized and tested for MPV. All mice were negative. However, this decreased the number of mice per group from the intended 40–35 (control), 36 (CR soy), 37 (CR fish), and 39 (CR lard).
Pathology
Gross necropsies were performed on 33 control, 34 CR soy, 39 CR lard, and 34 CR fish animals. Errors in animal processing or severe autolysis prevented necropsies from being completed on 2 control, 2 CR soy, and 3 CR fish mice. In addition to gross necropsies, liver, kidneys, brain, lungs and upper respiratory tract, spleen, pancreas, reproductive tract, heart and great vessels, thymus, lymph nodes, gastrointestinal tract, urinary tract, haired skin, ears, eyes, and skeletal muscle were processed for histologic examination. Other tissues were also processed for examination if gross lesions were observed at the time of necropsy. Tissue samples were immersion-fixed in 10% neutral buffered formalin. Formalin-fixed samples were routinely processed, embedded in paraffin, cut into 5 μm-thick sections and stained with hematoxylin and eosin.
Statistical Analysis
Differences in maximum and mean body weights for each group were assessed by an Analysis of Variance (ANOVA). Pairwise differences between diets were studied using a Tukey’s Honestly Significant Difference procedure. A Type I error rate of 0.05 was used to determine significance for all hypothesis tests.
We estimated survival curves for each diet group using the Kaplan-Meier method and tested for differences in survival among the groups with a log rank test. We tested all pairwise comparisons with log rank tests and a Bonferroni adjustment to maintain the family-wise Type I error rate at 0.05. The potential effect of body weight on longevity in addition to diet was evaluated with a Cox proportional hazard model. Body weight was incorporated into the modeling in several ways. First, we used body weight as a time-invariant covariate, and included the maximum or mean body weight over the study period. In a second analysis, we included body weight as time varying covariate. The last recorded weight was used as the weight at the time of death. Proc PHREG in SAS Version 9.3 was then used to fit a Cox proportional hazard model evaluating the effect of diet with adjustment for body weight.
We conducted additional analyses to better understand where the survival curves differed. We generalized Sprent’s (32) test for equality of medians to test for equality of survival times over a range of percentiles. Specifically, we tested whether selected percentiles of survival times differed among the diet groups for all pairwise comparisons.
Results
Body Weight
Weekly body weights for the mice in the four diet groups are shown in Figure 1. Regardless of the dietary fat source, 40% CR significantly decreased maximum and mean body weight (p < .001) when compared to the Control mice (Table 2). However, there were no differences in mean or maximum body weights between the three 40% CR groups (Table 2).
Figure 1.
Mean body weights in male C57BL/6J mice fed Control (5% CR soybean oil) or 40% calorie restricted diets (CR fish oil, CR soybean oil, CR lard) across the life span.
Table 2.
Maximum Body Weight and Body Weight Averaged Across Life Span in Male C57BL/6J Mice Consuming a Control Diet (5% CR soybean oil) or 40% Calorie Restricted Diets Containing Soybean Oil (CR-Soy), Lard (CR-Lard), or Fish Oil (CR-Fish)*
| Control 5% CR-Soy (n = 35) | 40% CR-Soy (n = 36) | 40% CR-Lard (n = 39) | 40% CR-Fish (n = 37) | |
|---|---|---|---|---|
| Maximum body weight (g) | 40.04±2.85 | 32.74±2.78† | 32.00±3.23† | 33.85±2.50† |
| Average body weight (g) | 32.94±2.66 | 23.43±1.14† | 23.38±0.86† | 24.28±1.08† |
Note: *Data are presented as means ± standard deviations.
†p < .05, based on Tukey’s Honestly Significant Difference multiple comparison procedure.
Longevity
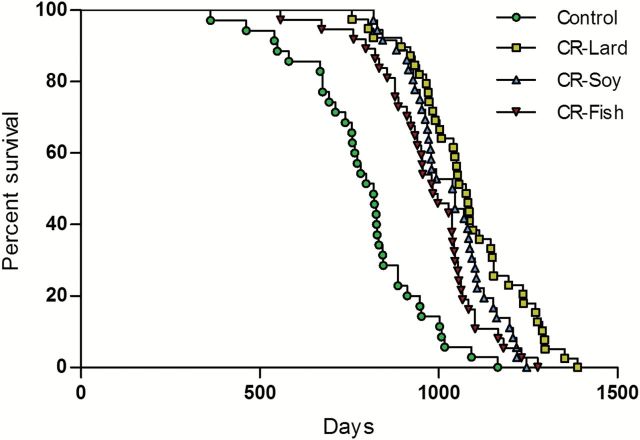
The survival curves for the mice in the four diet groups are shown in Figure 2. Life span was increased (p < .02) in all three CR groups compared to the Control group. Median life span was increased (p < .001) by 31.7% (CR lard), 27.5% (CR soy), and 20.3% (CR fish) in the CR mice compared to the control animals (Table 3).
Figure 2.
Survival curves for male C57BL/6J mice fed Control (5% CR soybean oil) or 40% calorie restricted diets (CR fish oil, CR soybean oil, CR lard).
Table 3.
Life Span Statistics for Male C57BL/6J Mice Consuming a Control Diet (5% CR soy bean oil) or 40% Calorie Restricted Diets Containing Soybean Oil (CR-Soy), Lard (CR-Lard) or Fish Oil (CR-Fish)*
| Percentile | Control 5% CR-Soy (n = 35) | 40% CR-Soy (n = 36) | 40% CR-Lard (n = 39) | 40% CR-Fish (n = 37) |
|---|---|---|---|---|
| 70th | 739 (649, 829) | 963 (930, 996) | 990 (948, 1,032) | 922 (844, 1,000) |
| 50th | 818 (757, 879) | 1039 (942, 1,136) | 1077 (1,034, 1,120) | 984 (896, 1,072) |
| 30th | 845 (784, 906) | 1102 (1,074, 1,130) | 1154 (1,067, 1,241) | 1,045 (1,022, 1,068) |
| 10th | 1,008 (915, 1,101) | 1,208 (1,165, 1,251) | 1,296 (1,243, 1,349) | 1,166 (1,070, 1,262) |
| Longest Lived | 1,165 | 1,246 | 1,389 | 1,277 |
Note: *Survival times (days) with approximate 95% confidence interval indicated in parenthesis.
Among the CR groups, survival was increased (p < .05) in the CR lard group compared to either the CR Soy or CR fish groups (Figure 2). There were no significant differences in the survival curves between the CR Soy and CR fish animals. There was an increase (p < .03) in median life span, 70th percent survival, 30th percent survival and a strong trend (p = .057) toward an increase in 10th percent survival in the CR lard versus CR fish group (Table 3). In contrast, the differences in survival between the CR lard and CR Soy groups were smaller in magnitude and not significant after adjusting for multiple comparisons among the four groups.
Cox proportional hazard analysis yielded similar survival differences between groups as those obtained using the Kaplan-Meier method and log-rank test (Table 4). Body weight was incorporated into the model as either a time-variant or time-varying variable but was not significant in any of these analyses. Diet group remained a significant predictor of survival in all of these analyses.
Table 4.
Estimated Hazard Ratios From Cox Proportional Model for Comparison Among Male C57BL/6J Mice Consuming a Control Diet (5% CR soy bean oil) or 40% Calorie Restricted Diets Containing Soybean Oil (CR-Soy), Lard (CR-Lard) or Fish Oil (CR-Fish)
| Description | Estimate | 95% Wald confidence limits | |
|---|---|---|---|
| Control vs CR-Soy | 3.978* | 1.879 | 4.858 |
| CR-Lard vs CR-Soy | 0.612* | 0.378 | 0.990 |
| CR-Fish vs CR-Soy | 1.316 | 0.830 | 2.089 |
| CR-Fish vs CR-Lard | 2.153* | 1.336 | 3.469 |
Note: *Significant differences for the indicated comparison
Pathology
The necropsy results for the four diet groups are summarized in Table 5 and Supplementary Tables 1 and 2. Necropsies were performed on 33 control, 34 CR soy, 39 CR lard, and 34 CR fish animals. Errors in animal processing or severe autolysis prevented necropsies from being completed on 2 control, 2 CR soy, and 3 CR fish mice. Statistical comparisons between diet groups were only performed for a few pathology findings since the low frequency of most pathology findings precluded these comparisons.
Table 5.
Prevalence of Neoplasms in Male C57BL/6J Mice Consuming a Control Diet (5% CR soy bean oil) or 40% Calorie Restricted Diets Containing Soybean Oil (CR-Soy), Lard (CR-Lard) or Fish Oil (CR-Fish)
| Control 5% CR-Soy (n = 35) | 40% CR-Soy (n = 36) | 40% CR-Lard (n = 39) | 40% CR-Fish (n = 37) | |
|---|---|---|---|---|
| Sarcoma | 13 | 19 | 24 | 15 |
| Lymphoma | 1 | 0 | 2 | 3 |
| Carcinoma | 1 | 1 | 1 | 0 |
| Nephroma | 4 | 5 | 5 | 7 |
| Adenoma (other than nephroma) | 9 | 1 | 4 | 6 |
| Adenocarcinoma | 1 | 0 | 0 | 0 |
| Leiomyosarcoma | 1 | 0 | 1 | 0 |
| Osteofibroma | 0 | 0 | 1 | 0 |
| Leukemia | 1 | 0 | 0 | 0 |
| Total mice with any kind of neoplasm | 26 | 23 | 30 | 23 |
The most frequent cause of death in all diet groups was hepatic histiocytic sarcoma, which was present in 65 animals. Nephromas (n = 21) and adenomas (n = 19), mainly pulmonary adenomas, also occurred at a high incidence. Calorie restriction by itself (CR soy vs Control) or dietary fat composition in the CR groups did not significantly alter cancer incidence, including total neoplasm occurrence or incidence of specific cancer types.
In comparisons between CR-soy and Control mice, mineralization in brain, testes, myocardium, and great vessels (especially aorta and pulmonary arteries) was significantly (p = .003) more frequent in CR-Soy animals. Great vessel mineralization was absent from the Control group, but was detected in 41%–50% of the CR mice, regardless of dietary fat source. No differences were detected in tissue mineralization between any of the three CR groups. Other CR-related differences in pathology include an increase in testicular atrophy (33.3% in Control vs 71.8% in CR-Soy, p = .001) and a decrease in dilation of seminal vesicles (30.3% in Control-Soy vs 2.9% in CR-Soy, p = .003).
An increase (p = .047) in Kupffer cell hyperplasia in the CR-Fish compared to CR-Lard (7.7% vs 29.4%) group was the only pathology difference detected among the three CR diets.
Discussion
The results of the present study indicate that dietary lipid composition influences life span in CR mice. In particular, life span was increased in CR mice consuming a diet with a high proportion of monounsaturated and saturated fatty acids compared to CR mice consuming diets with high proportions of n-3 or n-6 PUFAs. There are several possible mechanisms through which dietary fatty acids could modulate life span in CR animals.
Membrane Phospholipids and Oxidative Stress
The membrane theory of aging proposes that life span is inversely related to the level of unsaturation, and in particular the level of n-3 polyunsaturated fatty acids, in membrane phospholipids (6, 33–35). It has also been proposed that a decrease in long-chain polyunsaturated fatty acids in mitochondrial membrane phospholipids may be a mechanism contributing to the anti-aging effects of CR (7–9). In previous studies, we showed that liver (21) and skeletal muscle (18, 28) mitochondrial phospholipid fatty acid composition was altered in CR mice in a manner that reflects the unsaturated fatty acid composition of the diet. In particular, CR fish oil mice had an increased unsaturation index and higher levels of n-3 fatty acids in mitochondrial phospholipids than mice consuming either CR lard or soybean oil diets (18, 21, 28). Furthermore, the CR lard group had a decreased peroxidizability index compared to CR fish for skeletal muscle and both CR fish and CR soybean oil for liver mitochondrial phospholipids through 8 months of CR (Supplementary material). The life span mice consumed the same diets used in these cross-sectional studies, and thus, the mice on the three CR diets would be expected to have differences in mitochondrial membrane phospholipid composition which would impact life span if membrane fatty acid composition is a primary determinant of aging. The membrane theory of aging focuses on lipid peroxidation as the mechanism through which fatty acids influence life span. Consistent with this theory, the CR lard group had the lowest liver phospholipid peroxidizability index and the longest life span. However, the study did not provide overall support for the idea that membrane phospholipid fatty acid peroxidizability is the predominant mechanism through which dietary fatty acids influence lifespan. In particular, the CR soybean oil and CR fish oil groups had similar life spans despite the fact that peroxidizability index was significantly higher in liver and skeletal muscle mitochondria from the CR fish oil animals (Supplementary material). Similarly, it has been reported that life span was not different between ad libitum fed C57BL/6J mice consuming diets with either fish oil or sunflower oil (high in n-6 fatty acids) as the primary lipid source, despite the fact that peroxidizability index was significantly increased in liver and heart phospholipids from the mice consuming fish oil (36). Taken together, the results of these studies indicate that dietary fatty acids do not modulate lifespan in mice primarily by changing membrane composition to decrease susceptibility to peroxidation.
Membrane Proteins and Membrane-Linked Processes
Mitochondrial phospholipid fatty acids influence the activity of membrane proteins (10–14), membrane permeability (15–17), ROS production (18–20) and other membrane-linked processes. Thus, it is possible that dietary fatty acids could impact life span by altering membrane function through changes in phospholipid fatty acid composition. A comparative study investigating the relationship between membrane phospholipid fatty acids and life span concluded that the influence of the ratio of n-3 to n-6 PUFAs on life span was due to membrane effects distinct from simply changing membrane peroxidizability (37). Using the same diets as the present study, we have shown that capacity for mitochondrial complex III to produce ROS is reduced in the CR lard compared to other diet groups at 8 months of CR (28). This decrease in capacity for ROS production could be a factor contributing to the increase in life span observed in the CR lard mice. In other CR studies using the same diets as the present study, we have also shown that mitochondrial ultrastructure (29), apoptotic signaling (30), membrane proton permeability, and electron transport chain enzyme activities (18, 21) are altered by dietary fatty composition. However, these changes appear to depend on tissue and age (or duration of CR). Additional studies are needed to determine which of these membrane-linked processes may contribute to dietary lipid-induced changes in life span in CR animals.
Gene Transcription
Dietary fatty acids play an important role in regulating gene expression (38–40) and it is possible that this function of fatty acids also has an impact on life span. In particular, n-3 and n-6 PUFAs have been shown to be ligands for peroxisome proliferator-activated receptors (40, 41). Several saturated fatty acids also bind to peroxisome proliferator-activated receptors, although their relevance as peroxisome proliferator-activated receptor ligands at physiological concentrations is still open for debate (41). Peroxisome proliferator-activated receptors play an important role in regulating the transcription of genes involved in fatty acid metabolism and inflammation (41, 42), and there is growing appreciation of the role fatty acid-related changes in gene transcription may play in disease processes. However, there is currently little information about the influence of dietary fatty acids on gene transcription in CR animals. Further work is needed to determine if changes in gene transcription are a viable mechanism through which dietary fatty acids may modulate life span response to CR.
Pathology
Consistent with previous studies in C57BL/6J mice (43–45), neoplasms were the primary cause of death with histiocytic sarcoma as the major neoplasm in all four diet groups. There were no differences in prevalence of neoplasms or other major measures of end-of-life pathology between the three CR diet groups. Thus, differences in life span between the CR lard mice and the other CR groups were likely due to delay in onset of disease rather than preventing the occurrence of specific disease conditions. However, a clear limitation of the present study is that measures of pathology were only completed at the end of life, and additional cross-sectional studies are needed to truly determine if dietary fat composition influences the onset of disease in CR mice.
In the present study, there was no difference in incidence of cancer between the control and CR groups. While many studies have reported that CR decreases incidence of cancer (reviewed in (46, 47)), this finding has not been universal (45, 48). The reason for the difference in incidence of cancer between CR studies is not clear, however, it is possible that the 5% CR used in the control group may have mitigated differences between groups in the present study.
An unexpected finding of the present study was that mineralization of the great vessels, heart, testes, and thalamus were increased in the CR groups compared to the control animals. This mineralization was not simply due to diet alone since the CR soy and control group were consuming the same diet and very few control animals showed signs of mineralization. The results suggest that very advanced age, as achieved by many CR animals, may be required for noticeable signs of mineralization (and other age-related lesions). The mineralization observed in the CR mice was not of sufficient magnitude to suggest that it was a cause of death. Soft tissue mineralization, especially of the heart and thalamus, are recognized changes with aging (49). However, large vessel mineralization is not a typical aging change but is associated with atherogenic diets or pre-existing atherosclerosis (50), although there was no evidence of atherosclerosis in any of the CR groups in the present study. Future studies using AIN-93M diets for long-term studies in CR mice should assess tissue mineralization to determine if this is a common pathology with this diet at very advanced age.
The Optimum Fat for CR Diets
It is generally accepted that the retardation of aging with CR is due to a decrease in energy intake rather than a decrease in the amount of any particular nutrient (51), however, little is known about whether there is an optimum diet composition for promoting long life in CR animals. A number of diets, with a range of ingredients, have been used for CR and aging studies (52), but so far few studies have attempted to compare diets to ascertain if dietary composition influences life span in CR mice. The results of the present study indicate life span in male CR mice is sensitive to diet lipid composition. In particular, life span was increased in mice consuming a CR diet containing lard compared to animals consuming CR diets with fish oil or soybean oil. This result seems at odds with the reported health benefits of fish oil, including decreased inflammation (42, 53) and fat mass (54–58) and increased mitochondrial biogenesis (59) and capacity for fatty acid oxidation (60, 61). However, CR induces many of the same physiological changes as n-3 fatty acids, including decreased ROS production (3, 4), adiposity (62, 63) and inflammation (64, 65), and increased fatty acid oxidation (66). Thus, it is possible that n-3 fatty acids may not be able to induce additional changes in CR animals and the positive health effects of these fatty acids may be dampened or lost when coupled with CR. There is some evidence that consumption of diets with a high proportion of saturated and monounsaturated fatty acids may increase mitochondrial electron transport chain activity in old animals (67). It has also been reported that mitochondrial ROS production and oxidative damage (68–70) are decreased and mitochondrial function (69) is improved in animals consuming diets with a high proportion of monounsaturated versus polyunsaturated fatty acids. It is possible that diets with a high proportion of saturated and/or monounsaturated fatty acids may show clear benefits in mice that are not overweight and have low levels of inflammation. The present study raises the possibility that the optimum fatty acids for health and life span may be different for ad libitum fed versus CR animals.
A limitation of the present study, and all studies using complex dietary lipid sources, is that various dietary lipids differ in multiple fatty acids. Studies using diets with purified fatty acids are likely required to identify the specific fatty acids which influence life span in CR mice.
It is important to note that the goal of the present study was to determine if dietary lipid composition alters life span in a groups of mice fed at a common level of energy intake. One control (5% CR soybean oil) was included for the standard AIN93M diet to demonstrate that 40% CR produced the expected life span extension compared to 5% CR. However, the studies were not capable of determining if dietary lipid composition alters the magnitude of life span extension with CR in mice consuming the same diet at two or more levels of energy intake. Additional studies are needed to determine if the increased life span in 40% CR mice consuming the lard diet would also be observed at other levels of energy intake.
The life span of the control mice in the present study were comparable to the life spans previously reported for C57BL/6 mice consuming AIN-93G diets. However, the life spans of the mice, and especially the control group, were a few months shorter than the life spans reported in a few studies for C57BL/6 mice consuming non-purified diets (44, 45, 71). Although the focus of the present study was on dietary lipid composition, it is possible that composition of protein, carbohydrates, or other dietary components may also influence life span. Studies comparing various diets under identical conditions in animals maintained on specific levels of energy intake are needed to truly determine the extent to which various diets influence life span in animals maintained on CR.
Conclusion
The results of the present study indicate that dietary fatty acids influence life span in CR mice. In particular, a diet with a high proportion of monounsaturated and saturated fatty acids promotes a longer life span in CR animals maintained at a common energy intake (8.6 kcal/d) than diets containing a high proportion of unsaturated fatty acids. The results of this study indicate that dietary fat source should be considered when designing a diet to optimize life span in CR mice.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Health (www.nia.nih.gov) (RO1AG028125).
Supplementary Material
References
- 1. Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. 10.1016/j.mam.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 2. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. [DOI] [PubMed] [Google Scholar]
- 3. Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–3717. 10.1210/en.2005-0378 [DOI] [PubMed] [Google Scholar]
- 4. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. 10.1126/science.273.5271.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hulbert AJ. Life, death and membrane bilayers. J Exp Biol. 2003;206:2303–2311. 10.1242/jeb.00399 [DOI] [PubMed] [Google Scholar]
- 6. Pamplona R, Barja G, Portero-Otín M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann N Y Acad Sci. 2002;959:475–490. 10.1111/j.1749-6632.2002.tb02118.x [DOI] [PubMed] [Google Scholar]
- 7. Laganiere S, Yu BP. Effect of chronic food restriction in aging rats. II. Liver cytosolic antioxidants and related enzymes. Mech Ageing Dev. 1989;48:221–230. [DOI] [PubMed] [Google Scholar]
- 8. Laganiere S, Yu BP. Modulation of membrane phospholipid fatty acid composition by age and food restriction. Gerontology. 1993;39:7–18. 10.1159/000213509 [DOI] [PubMed] [Google Scholar]
- 9. Yu BP, Lim BO, Sugano M. Dietary restriction downregulates free radical and lipid peroxide production: plausible mechanism for elongation of life span. J Nutr Sci Vitaminol. 2002;48:257–264. [DOI] [PubMed] [Google Scholar]
- 10. Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985;822:1–42. [DOI] [PubMed] [Google Scholar]
- 11. Dowhan W, Mileykovskaya E, Bogdanov M. Diversity and versatility of lipid-protein interactions revealed by molecular genetic approaches. Biochim Biophys Acta. 2004;1666:19–39. 10.1016/j.bbamem.2004.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Innis SM, Clandinin MT. Dynamic modulation of mitochondrial membrane physical properties and ATPase activity by diet lipid. Biochem J. 1981;198:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. 10.1016/j.bbamem.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 14. Marsh D. Protein modulation of lipids, and vice-versa, in membranes. Biochim Biophys Acta. 2008;1778:1545–1575. 10.1016/j.bbamem.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 15. Brand MD, Couture P, Hulbert AJ. Liposomes from mammalian liver mitochondria are more polyunsaturated and leakier to protons than those from reptiles. Comp Biochem Physiol Biochem Mol Biol. 1994;108:181–188. [DOI] [PubMed] [Google Scholar]
- 16. Brookes PS, Buckingham JA, Tenreiro AM, Hulbert AJ, Brand MD. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:325–334. [DOI] [PubMed] [Google Scholar]
- 17. Porter RK, Hulbert AJ, Brand MD. Allometry of mitochondrial proton leak: influence of membrane surface area and fatty acid composition. Am J Physiol. 1996;271:R1550–R1560. [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Hagopian K, McDonald RB, et al. The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction. J Gerontol A Biol Sci Med Sci. 2012;67:1121–1131. 10.1093/gerona/gls113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hagopian K, Weber KL, Hwee DT, et al. Complex I-associated hydrogen peroxide production is decreased and electron transport chain enzyme activities are altered in n-3 enriched fat-1 mice. PLoS One. 2010;5:e12696. 10.1371/journal.pone.0012696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramsey JJ, Harper ME, Humble SJ, et al. Influence of mitochondrial membrane fatty acid composition on proton leak and H2O2 production in liver. Comp Biochem Physiol B Biochem Mol Biol. 2005;140:99–108. 10.1016/j.cbpc.2004.09.016 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Hagopian K, Bibus D, et al. The influence of dietary lipid composition on liver mitochondria from mice following 1 month of calorie restriction. Biosci Rep. 2013;33:83–95. 10.1042/BSR2012006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Umezawa M, Takeda T, Kogishi K, et al. Serum lipid concentrations and mean life span are modulated by dietary polyunsaturated fatty acids in the senescence-accelerated mouse. J Nutr. 2000;130:221–227. [DOI] [PubMed] [Google Scholar]
- 23. Tsuduki T, Honma T, Nakagawa K, Ikeda I, Miyazawa T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition. 2011;27:334–337. 10.1016/j.nut.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 24. Berdanier CD, Johnson B, Hartle DK, Crowell W. Life span is shortened in BHE/cdb rats fed a diet containing 9% menhaden oil and 1% corn oil. J Nutr. 1992;122:1309–1317. [DOI] [PubMed] [Google Scholar]
- 25. Takeuchi H, Sekine S, Noguchi O, Murano Y, Aoyama T, Matsuo T. Effect of life-long dietary n-6/n-3 fatty acid ratio on life span, serum lipids and serum glucose in Wistar rats. J Nutr Sci Vitaminol (Tokyo). 2009;55:394–399. [DOI] [PubMed] [Google Scholar]
- 26. Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J Immunol. 2010;184:5280–5286. 10.4049/jimmunol.0903282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jolly CA, Muthukumar A, Avula CP, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB x NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr. 2001;131:2753–2760. [DOI] [PubMed] [Google Scholar]
- 28. Chen Y, Hagopian K, Bibus D, et al. The influence of dietary lipid composition on skeletal muscle mitochondria from mice following eight months of calorie restriction. Physiol Res. 2014;63:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khraiwesh H, López-Domínguez JA, López-Lluch G, et al. Alterations of ultrastructural and fission/fusion markers in hepatocyte mitochondria from mice following calorie restriction with different dietary fats. J Gerontol A Biol Sci Med Sci. 2013;68:1023–1034. 10.1093/gerona/glt006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. López-Domínguez JA, Khraiwesh H, González-Reyes JA, et al. Dietary fat modifies mitochondrial and plasma membrane apoptotic signaling in skeletal muscle of calorie-restricted mice. Age (Dordr). 2013;35:2027–2044. 10.1007/s11357-012-9492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. NRC. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academies Press; 1995. [PubMed] [Google Scholar]
- 32. Sprent P. Applied Non-parametric Statistical Methods. 2nd ed. London: Chapman and Hall; 1993. [Google Scholar]
- 33. Hulbert AJ. On the importance of fatty acid composition of membranes for aging. J Theor Biol. 2005;234:277–288. 10.1016/j.jtbi.2004.11.024 [DOI] [PubMed] [Google Scholar]
- 34. Hulbert AJ. The links between membrane composition, metabolic rate and lifespan. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:196–203. 10.1016/j.cbpa.2006.05.014 [DOI] [PubMed] [Google Scholar]
- 35. Pamplona R, Portero-Otín M, Riba D, et al. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J Lipid Res. 1998;39:1989–1994. [PubMed] [Google Scholar]
- 36. Valencak TG, Ruf T. Feeding into old age: long-term effects of dietary fatty acid supplementation on tissue composition and life span in mice. J Comp Physiol B. 2011;181:289–298. 10.1007/s00360-010-0520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valencak TG, Ruf T. N-3 polyunsaturated fatty acids impair lifespan but have no role for metabolism. Aging Cell. 2007;6:15–25. 10.1111/j.1474-9726.2006.00257.x [DOI] [PubMed] [Google Scholar]
- 38. Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci. 2004;41:41–78. 10.1080/10408360490278341 [DOI] [PubMed] [Google Scholar]
- 39. Legrand P, Rioux V. The complex and important cellular and metabolic functions of saturated fatty acids. Lipids. 2010;45:941–946. 10.1007/s11745-010-3444-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Price PT, Nelson CM, Clarke SD. Omega-3 polyunsaturated fatty acid regulation of gene expression. Curr Opin Lipidol. 2000;11:3–7. [DOI] [PubMed] [Google Scholar]
- 41. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007–1022. 10.1016/j.bbadis.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. 10.3945/jn.111.155259 [DOI] [PubMed] [Google Scholar]
- 43. Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol. 1995;23:570–582. [DOI] [PubMed] [Google Scholar]
- 44. Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. 10.1093/gerona/60.12.1510 [DOI] [PubMed] [Google Scholar]
- 45. Ramsey JJ, Tran D, Giorgio M, et al. The Influence of Shc Proteins on Life Span in Mice. J Gerontol A Biol Sci Med Sci. 2014;69:1177–1185. :10.1093/gerona/glt198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meynet O, Ricci JE. Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol Med. 2014;20:419–427. 10.1016/j.molmed.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 47. Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- 48. Pugh TD, Oberley TD, Weindruch R. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res. 1999;59:1642–1648. [PubMed] [Google Scholar]
- 49. Frith CH, Ward JM. Color Atlas of Neoplastic and Non-neoplastic Lesions in Aging Mice. New York: Elsevier; 1988. [Google Scholar]
- 50. Plendl J, Kolle S, Sinowatz F, Schmahl W. Nonneoplastic lesions of blood vessels. In: Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, eds. Pathobiology of the Aging Mouse. Washington, DC: ILSI Press; 1996:387. [Google Scholar]
- 51. Masoro EJ. Caloric restriction and aging: controversial issues. J Gerontol A Biol Sci Med Sci. 2006;61:14–19. [DOI] [PubMed] [Google Scholar]
- 52. Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. [DOI] [PubMed] [Google Scholar]
- 53. Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baillie RA, Takada R, Nakamura M, Clarke SD. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fatty Acids. 1999;60:351–356. [DOI] [PubMed] [Google Scholar]
- 55. Hill JO, Peters JC, Lin D, Yakubu F, Greene H, Swift L. Lipid accumulation and body fat distribution is influenced by type of dietary fat fed to rats. Int J Obes Relat Metab Disord. 1993;17:223–236. [PubMed] [Google Scholar]
- 56. Jones PJ. Effect of fatty acid composition of dietary fat on energy balance and expenditure in hamsters. Can J Physiol Pharmacol. 1989;67:994–998. [DOI] [PubMed] [Google Scholar]
- 57. Su W, Jones PJ. Dietary fatty acid composition influences energy accretion in rats. J Nutr. 1993;123:2109–2114. [DOI] [PubMed] [Google Scholar]
- 58. Tsuboyama-Kasaoka N, Sano K, Shozawa C, Osaka T, Ezaki O. Studies of UCP2 transgenic and knockout mice reveal that liver UCP2 is not essential for the antiobesity effects of fish oil. Am J Physiol Endocrinol Metab. 2008;294:E600–E606. 10.1152/ajpendo.00551.2007 [DOI] [PubMed] [Google Scholar]
- 59. Flachs P, Horakova O, Brauner P, et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia. 2005;48:2365–2375. 10.1007/s00125-005-1944-7 [DOI] [PubMed] [Google Scholar]
- 60. Halminski MA, Marsh JB, Harrison EH. Differential effects of fish oil, safflower oil and palm oil on fatty acid oxidation and glycerolipid synthesis in rat liver. J Nutr. 1991;121:1554–1561. [DOI] [PubMed] [Google Scholar]
- 61. Ide T, Kobayashi H, Ashakumary L, et al. Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. Biochim Biophys Acta. 2000;1485:23–35. [DOI] [PubMed] [Google Scholar]
- 62. Ramsey JJ, Hagopian K. Energy expenditure and restriction of energy intake: could energy restriction alter energy expenditure in companion animals? J Nutr. 2006;136(7 Suppl):1958S–1966S. [DOI] [PubMed] [Google Scholar]
- 63. Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. 10.1016/j.mam.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 64. Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327–335. 10.1111/j.1749-6632.2001.tb05662.x [PubMed] [Google Scholar]
- 65. Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol. 2009;44:41–45. 10.1016/j.exger.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2010;298:E108–E116. 10.1152/ajpendo.00524.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bronnikov GE, Kulagina TP, Aripovsky AV. Dietary supplementation of old rats with hydrogenated peanut oil restores activities of mitochondrial respiratory complexes in skeletal muscles. Biochemistry (Mosc). 2010;75:1491–1497. [DOI] [PubMed] [Google Scholar]
- 68. Huertas JR, Martinez-Velasco E, Ibáñez S, et al. Virgin olive oil and coenzyme Q10 protect heart mitochondria from peroxidative damage during aging. Biofactors. 1999;9:337–343. [DOI] [PubMed] [Google Scholar]
- 69. Mataix J, Ochoa JJ, Quiles JL. Olive oil and mitochondrial oxidative stress. Int J Vitam Nutr Res. 2006;76:178–183. 10.1024/0300-9831.76.4.178 [DOI] [PubMed] [Google Scholar]
- 70. Mujahid A, Akiba Y, Toyomizu M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297:R690–R698. 10.1152/ajpregu.90974.2008 [DOI] [PubMed] [Google Scholar]
- 71. Yuan R, Meng Q, Nautiyal J, et al. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci USA. 2012;109:8224–8229. 10.1073/pnas.1121113109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.