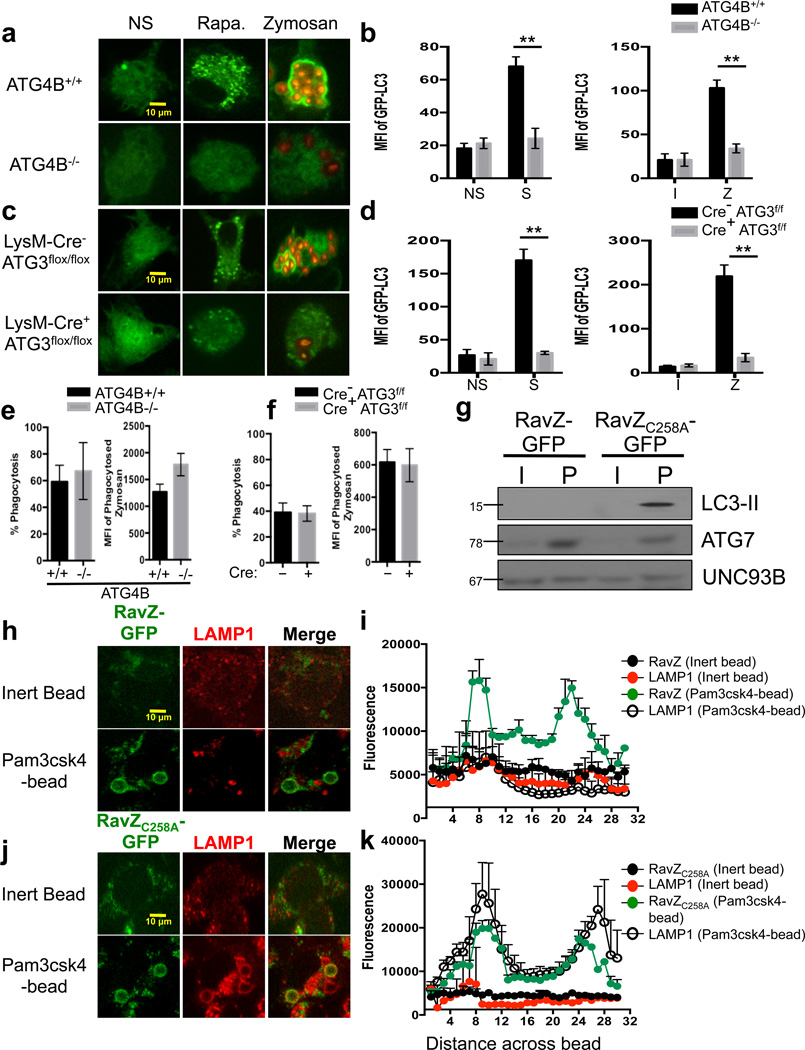
Figure 6. LAP-induced phagosome maturation requires LC3-II.
(a–b) Bone marrow-derived macrophages from ATG4B+/+ and ATG4B−/− mice were transfected with GFP-LC3. After 48 hours of transfection, cells were left untreated (NS) or were cultured with 200 nM rapamycin (Rapa., a), starvation conditions (S, b), Inert beads (I, b), or Alexa Fluor 594-zymosan (Zymosan or Z, a–b). (c–d) Bone marrow-derived macrophages from LysM-Cre− ATG3flox/flox and LysM-Cre+ ATG3flox/flox mice were transfected with GFP-LC3. After 48 hours of transfection, cells were left untreated (NS) or were cultured with 200 nM rapamycin (Rapa., c), starvation conditions (S, d), Inert beads (I, d), or Alexa Fluor 594-zymosan (Zymosan or Z, c–d). GFP-LC3 puncta was assessed at 18 h, and translocation of GFP-LC3 to the LAPosome was assessed at 1 h by confocal microscopy (a, c) and flow cytometry (b, d). Data are presented as mean ± SD of three independent experiments (**p < 0.001). (e–h) RAW cells were transfected with RavZ-GFP (e–f) or RavZC258A-GFP (g–h). After 48 hours of transfection, cells were fed inert beads or Pam3csk4-beads for 1 hour. Immunofluorescent staining was performed for LAMP1 and analyzed by microscopy. Representative images (e, g) and signal intensity profiles (f, h) for RavZ, RavZC258A, and LAMP1 across phagocytosed beads are quantified and shown graphically (n ≥ 20 / genotype). Data are presented as mean ± SD of two independent experiments. (i) RAW cells were transfected with RavZ-GFP or RavZC258A-GFP. After 48 hours of transfection, cells were allowed to phagocytose inert beads or Pam3csk4-beads for 1 hour. Phagosomes were purified using sucrose gradient as described in experimental procedures. Phagosome proteins (left), as well as whole cell lysates from non-stimulated cells (right), were solubilized in SDS-PAGE and blotted with the indicated antibodies. The results presented are representative of three independent experiments.