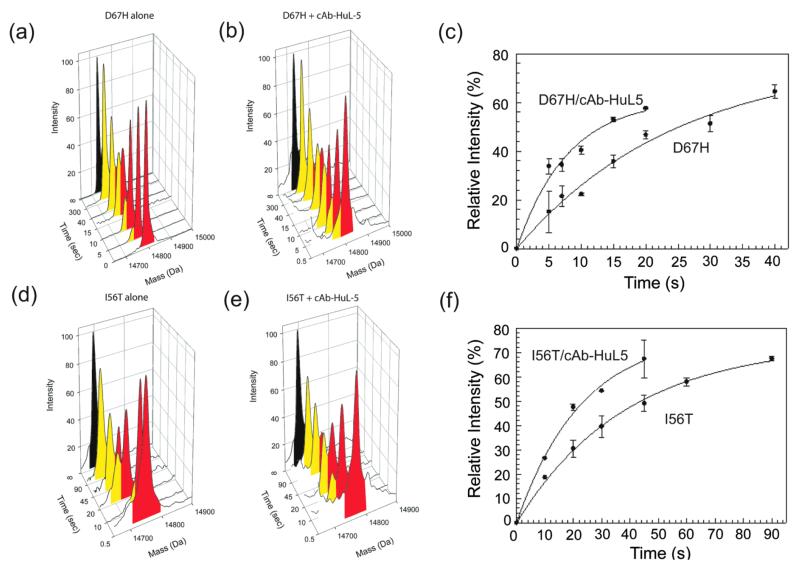
Figure 4.
H/D exchange behavior of the I56T and D67H variants of human lysozyme in the absence and presence of cAb-HuL5. Electrospray mass spectra of the D67H (a, b) and I56T (d, e) variants following H/D exchange in the absence (a, d) and presence (b, e) of cAb-HuL5 at pH 8.0 and 37 °C. For the lysozyme/nanobody mixtures, a 2-fold molar excess of cAb-HuL5 was added to ensure that effectively all the lysozyme molecules were in the bound state. The mass peaks colored red result from the gradual loss of deuterium atoms through an EX2 mechanism due to local independent structural fluctuations. The mass peaks colored yellow arise from locally cooperative unfolding events that give rise to H/D exchange through an EX1 mechanism. Note that the I56T and D67H variants exhibit bimodal distributions of mass peaks even in the presence of cAb-HuL5. Time courses of the relative intensities of the lower mass species (i.e., the yellow peaks) of the (c) D67H and (f) I56T variants in the absence and presence of cAb-HuL5. The data are the average of two experiments, and the bars show the error estimated from these measurements. These data were fitted to exponential functions to determine the time constants (τ) of the locally cooperative unfolding of the lysozyme variants; τD67H = 25.2 ± 5.1 s; τD67H/cAb-HuL5 = 7.9 ± 0.05 s; τI56T = 36.8 ± 3.2 s; τI56T/cAb-HuL5 = 24.4 ± 8.6 s.