Figure 2.
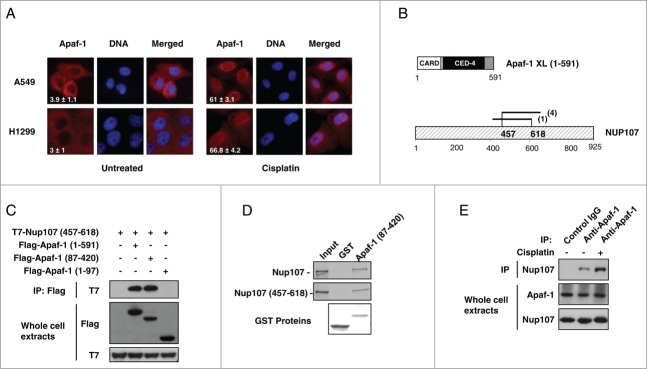
Apaf-1 associates with the nucleoporin Nup107. (A) Apaf-1 nuclear translocation upon genotoxic stress is independent of p53. A549 (p53+/+) and H1299 (p53−/−) cells were treated or not with cisplatin (20 μM, 24 h) and immunolabeled with anti-Apaf-1 antibody (red). Nuclei were stained with DAPI. Numbers indicate percentage of cells with nuclear Apaf-1 (means ± s.e.m.); n = 3. (B) Top: Schematic representation of Apaf-1 XL (1-591), which was used as bait for the yeast 2-hybrid screening. Bottom: Overlapping fragments of the nucleoporin Nup107 (NM_020401) that interact with Apaf-1 in the yeast-2-hybrid assay. Black lines indicate the fragments of Nup107 that interact with Apaf-1. The number of times the fragments were identified in the screen are indicated. The minimal interacting domain is from aa 457 to aa 618. (C) Nup107 (457–618) interacts with the CED-4 domain of Apaf-1. 293T cells were cotransfected with T7-Nup107 (457–618) together with the indicated Apaf-1 Flag-tagged constructs. Cells were lysed and the lysates were subjected to anti-Flag immunoprecipitation (IP) followed by immunoblotting (IB) with anti-T7. The expression of different T7- or Flag-tagged constructs was determined by direct immunoblotting. (D) In vitro interaction of Apaf-1 with Nup107. 35S-labeled Nup107 or Nup107 (457-618) (lane 1, 10% input) were incubated with an equal amount of glutathione Sepharose beads bound to an equal amount of GST (lane 2) or GST- Apaf-1 (87-420) (lane 3). Bound proteins were eluted and analyzed by SDS-PAGE followed by autoradiography. (E) Endogenous Apaf-1 interacts with endogenous Nup107. Whole cell extracts derived from A549 cells treated or not with cisplatin were immunoprecipitated with a control antibody or anti-Apaf-1 antibody and analyzed by immunoblotting with anti-Nup107 antibody.
