Abstract
Objective
To identify predictors of response to tumor necrosis factor (TNF) antagonists in ankylosing spondylitis (AS) and psoriatic arthritis (PsA).
Methods
Systematic review and meta-analysis of clinical trials and observational studies based on a systematic search. Meta-analyses of similar observations were performed using random effects computing summary OR. Heterogeneity was tested using I2, and risks of bias using funnel plots and the Egger test. Meta-regression was used to explore causes of heterogeneity.
Results
The electronic search captured 1340 references and 217 abstracts. 17 additional articles were identified after searching by hand. A total of 59 articles meet the purpose of the study and were reviewed. 37 articles (33 studies) included 6736 patients with AS and 23 articles (22 studies) included 4034 patients with PsA. 1 article included data on AS and PsA. Age (OR (95% CI) 0.91 (0.84 to 0.99), I2=84.1%), gender (1.57 (1.10 to 2.25), I2=0.0%), baseline BASDAI (1.31 (1.09 to 1.57), I2=0.0%), baseline BASFI (0.86 (0.79 to 0.93), I2=24.9%), baseline dichotomous C reactive protein (CRP) (2.14 (1.71 to 2.68), I2=22.3%) and human leucocyte antigen B27 (HLA-B27) (1.81 (1.35 to 2.42), I2=0.0%) predict BASDAI50 response in AS. No factor was identified as a source of heterogeneity. Only meta-analysis of baseline BASFI showed risk of publication bias (Egger test, p=0.004). Similar results were found for ASAS criteria response. No predictors of response were identified in PsA.
Conclusions
Young age, male sex, high baseline BASDAI, low baseline BASFI, high baseline CRP and HLA-B27 predict better response to TNF antagonists in AS but not in PsA.
Keywords: Psoriatic Arthritis, Spondyloarthritis, Anti-TNF
Key messages.
At the group level, demographic, serological, clinical and genetic factors predict response to biological therapies in AS and PsA.
However, the individual predictive value of these variables is limited.
Introduction
Tumor necrosis factor (TNF) antagonists are a major advance in the treatment of patients with inflammatory arthritis. The efficacy and safety of these drugs has been supported by clinical trials.1–7 However, not all patients respond to these therapies and, furthermore, they are not exempt from serious adverse events. TNF antagonists are associated with increased risk of infections, including reactivation of tuberculosis and other opportunistic infections.8–10 In the past few years new therapies have been approved for the treatment of spondyloarthritis, increasing the therapeutic options for these patients.11 12 How best to use these drugs remains unclear. An ability to identify which patients would have a better response to each biological therapy may help minimise the risks and costs associated with these treatments. The development of predictors of response might identify responders and thus help with making therapeutic decisions in clinical practice.
Several clinical and serological markers of response to biologics have been identified in rheumatoid arthritis (RA).13–18 However, data about predictors of response in patients with ankylosing spondylitis (AS) or psoriatic arthritis (PsA) are limited. The main objective of this study is to summarise information regarding predictors of response to TNF antagonists in patients with AS and PsA.
Materials and methods
We performed a systematic literature review to identify all publications analysing predictors of response to TNF antagonists in patients with AS or PsA. The protocol of the review is available by email on request. PRISMA consensus was followed for the review and meta-analysis.19
Systematic literature research
Medline, Embase, Web of Knowledge and the Cochrane Library were searched for articles published between 1998 and April 2013. The search strategy focused on synonyms for disease, TNF antagonist, predictor and response, and was limited to articles published in English, Spanish, French, Italian or Portuguese (see online supplementary text). We also included abstracts online from 2001 to 2013 of the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) congresses.
Selection of articles
The selection criteria for articles and abstracts were: (1) studies in patients with a diagnosis of AS or PsA; (2) studies in patients treated with at least one TNF antagonist; (3) studies collecting data on predictor of response with some method of measurement; and (4) retrospective or prospective observational studies, or intervention studies. Two reviewers (JRM and AS) screened articles and abstracts for selection criteria independently, using a third reviewer (ES) for consensus. Once unrelated articles were excluded, the full report of all the selected studies was reviewed. Subsequently, articles not fulfilling all selection criteria were excluded. A table summarising the reasons for exclusion is included in the online supplementary material. A reverse search of included articles and a hand search of published clinical trials of TNF antagonist in AS or PsA, and of documents of the Food and Drug Administration (FDA) were also performed.
Data extraction
Data collected included publication details, study design, characteristics of patients, treatment, predictor and definition of response.
Risk of bias
We created an ad hoc checklist to analyse the risk of bias of included studies, containing 30 items with punctuation from 0 to 100 (from higher to lower risk). This checklist was based on the guidelines for assessing quality in prognostic studies on the basis of framework of potential biases proposed by Hayden et al20 (available on request).
Statistical analysis
Results were presented as summary effect measures grouped by predictor and by response definition. When a measure of association was not available, this was calculated from the available data. Meta-analyses were performed using a random-effects approach, with the DerSimonian and Laird method computing the summary OR.21 Meta-analysis was only planned if at least three studies or subanalyses with similar design were available. For each analysis the effect was plotted by the inverse of its SE to identify risk of publication bias, assessing visually the symmetry of funnel plots, and its statistical significance using the Egger test.22 Heterogeneity was tested as proposed by Higgins and Thompson using I2.23 24 An I2 value >40% was arbitrarily chosen to represent high levels of heterogeneity. If high statistical heterogeneity was present, possible explanations were investigated using sensitivity analysis and meta-regression. Meta-regression aimed to determine the contribution of time to assess response, number of patients, quality of data, time of disease duration, biological used, design of the study, and levels of evidence to the summary effect. A p<0.10 was considered significant in the meta-regression and p<0.05 in other analyses. Stata V.11.1 (Stata/IC 11.1 for Windows, StataCorp LP, Texas, USA) was used in all statistical analyses.
Results
The search identified a total of 1340 articles and 217 abstracts. After title/abstract screening, 125 articles were retrieved for full text review. After hand search and reverse search, 17 additional articles were included. A total of 83 articles were excluded after detailed review. Finally, 59 articles and abstracts were included in the present analysis (see online supplementary figure S1).
In 55 studies from these 59 documents, 10 770 patients were included (6736 with AS and 4034 with PsA). Thirty-seven articles (33 studies) included patients with AS1 25–60 and 23 (22 studies) patients with PsA.4 43 61–81 One of these articles included data about AS and PsA, and these data were analysed separately.43 Quality of data was ≥70% in 33 (60.0%) of the studies; 20 (60.6%) in studies of AS and 13 (59.0%) in studies of PsA (tables 1 and 2). Individual results are presented according to predictors and disease in online supplementary material (see online supplementary tables S1–S8).
Table 1.
Table of evidence of studies of AS
| Study | Biological | Design | Duration | N | Q | LE | Age* | DD* | Women (%) | HLAB27+ (%) | Prior biologics (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arends et al26 | IFX, ETN, ADA | OP | 24 | 220 | 0.91 | 2 | 42.9 | 15.0 | 31.0 | 81.0 | 0.0 |
| Arends et al27 | ETN | OP | 48 | 92 | 0.75 | 2 | 41.2 | 9.0† | 26.0 | 83.0 | 0.0 |
| Braun et al28 | IFX | RCT | 12 | 34 | 0.65 | 3 | 40.6 | 16.4 | 32.0 | 91.0 | 0.0 |
| Braun et al29 | ADA | OP | 12 | 1250 | 0.75 | 2 | 44.0 | 11.0 | 30.0 | 82.0 | 26.0 |
| Davis et al30 | ETN | RCT | 24 | 138 | 0.83 | 3 | 42.1 | 10.1 | 24.0 | 84.0 | 0.0 |
| de Vries et al31 | IFX, ETN | OP | 12 | 155 | 0.80 | 2 | 42.0 | 8.0† | 35.0 | 79.0 | 0.0 |
| Fagerli et al32 | NA | OR | 12 | 249 | 0.60 | 4 | 41.9 | 10.1 | 32.1 | 90.7 | 0.0 |
| Fagerli et al33 | NA | OR | 12 | 289 | 0.61 | 4 | 42.4 | 9.9 | 32.6 | 90.5 | 0.0 |
| FDA-103795/512325 | ETN | RCT | 24 | 138 | 0.65 | 3 | 42.1 | 10.0 | 24.0 | 84.0 | NA |
| Glintborg et al34 | IFX, ADA, ETN | OR | 24 | 842 | 0.76 | 4 | 41.0† | 5.0† | 28.0 | NA | 0.0 |
| Haibel et al35 | ADA | RCT | 52 | 46 | 0.65 | 3 | 37.4 | 7.5 | 54.3 | 67.3 | 2.1 |
| Huang et al36 | IFX | OP | 10 | 63 | 0.76 | 2 | 32.8 | 10.9 | 20.0 | 90.5 | 0.0 |
| Inman et al37 | GOL | RCT | 14 | 278 | 0.75 | 3 | 38.0 | 5.2† | 28.1 | 83.0 | 0.0 |
| Kim et al38 | IFX | OP | 22 | 23 | 0.60 | 2 | 41.4 | 8.7† | 17.3 | 100.0 | 0.0 |
| Kristensen et al39 | IFX, ETN, ADA | OP | 96 | 243 | 0.83 | 2 | 43.0 | 16.0 | 25.5 | NA | 0.0 |
| Lord et al40 | IFX, ETN, ADA | OP | 24 | 261 | 0.91 | 2 | 43.0† | 13.0† | 18.0 | NA | 0.0 |
| Luc et al41 | IFX, ETN, ADA | OR | 144 | 175 | 0.81 | 4 | 27.1 | 12.1 | 22.0 | 88.0 | 0.0 |
| Maria Lizzio et al42 | IFX | OP | 54 | 47 | 0.51 | 2 | 46.8 | 14.7 | NA | NA | NA |
| Morales-Lara et al43 | IFX | OP | 48 | 33 | 0.50 | 2 | NA | NA | NA | NA | NA |
| Mulleman et al44 | IFX | RCT | 14 | 26 | 0.66 | 3 | 44.1† | 4.2† | 23.0 | NA | 15.3 |
| Navarro-Compan et al45 | NA | OP | 12 | 20 | 0.55 | 2 | 42.4 | 6.8 | 14.0 | 83.3 | NA |
| Ottaviani et al46 | IFX | OR | 24 | 155 | 0.83 | 4 | 43.1† | 8.0† | 36.7 | 64.9 | NA |
| Pedersen et al47 | IFX, ETN, ADA | OP | 22 | 60 | 0.90 | 2 | 40.0† | 12.0† | 20.0 | 82.0 | 0.0 |
| Perez-Guijo et al48 | IFX | OP | 30 | 19 | 0.60 | 3 | 37.4 | 14.2 | NA | 100.0 | 0.0 |
| Ramiro et al49 | NA | OR | 12 | 197 | 0.75 | 4 | NA | NA | NA | NA | NA |
| Rudwaleit et al52 | IFX. ETN | RCT | 12 | 99 | 0.78 | 3 | 38.4 | 14.8 | 32.0 | 89.0 | 0.0 |
| Rudwaleit et al51 | ADA | OP | 12 | 1159 | 0.75 | 2 | NA | NA | NA | NA | NA |
| Rudwaleit et al53 | IFX, ETN | RCT | 12 | 46 | 0.78 | 3 | 38.1 | 14.6 | 34.8 | 89.1 | 0.0 |
| Rudwaleit et al50 | ADA | OP | 12 | 1250 | 0.75 | 2 | 44.0 | 11.0 | 30.0 | 82.0 | 26.0 |
| Seitz et al54 | IFX, ETN, ADA | OP | 24 | 22 | 0.68 | 2 | 38.9 | 12.2 | 13.0 | NA | NA |
| Sieper et al55 | ADA | RCT | 240 | 315 | 0.83 | 3 | 42.3 | 11.0 | 25.1 | 78.8 | 0.0 |
| Stone et al56 | IFX | OP | 52 | 22 | 0.83 | 3 | 37.9† | 8.7† | 18.1 | 100.0 | 0.0 |
| Tong et al57 | IFX, ETN | OP | 12 | 99 | 0.75 | 2 | 41.6 | 9.4 | 22.2 | 91.2 | 0,0 |
| van der Heijde et al58 | IFX | RCT | 24 | 201 | 0.61 | 3 | 40.0 | 7.7 | 21.9 | 86.5 | NA |
| van der Heijde et al1 | ADA | RCT | 24 | 208 | 0.61 | 3 | 41.7 | 11.3 | 24.5 | 78.4 | 0.0 |
| Visvanathan et al59 | IFX | RCT | 24 | 201 | 0.80 | 3 | 40.0 | 10.1 | 21.9 | 86.5 | 0.0 |
| Wagner et al60 | GOL | RCT | 14 | 76 | 0.83 | 3 | 39.8 | NA | 30.2 | 77.6 | 0.0 |
*Data are expressed in means (years).
†Data are expressed in medians.
ADA, adalimumab; DD, disease duration; ETN: etanercept; GOL, golimumab; IFX, infliximab; LE, level of evidence; N, number of patients; NA, not available; OP, observational prospective; OR, observational retrospective; Q, quality; RCT, randomised clinical trial.
Table 2.
Table of evidence of studies of PsA
| Study | Biologic | Design | Duration | N | Q | LE | Age* | DD* | Women (%) | HLAB27+(%) | Prior biologics (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antoni et al4 | IFX | RCT | 24 | 100 | 0.67 | 3 | 47.1 | 8.4 | 29.0 | NA | 0.0 |
| Chandran et al61 | NA | OP | 11 | 40 | 0.66 | 2 | 44.0 | 12.0 | 30.0 | NA | NA |
| Chimenti et al62 | ADA, ETN | RCT | 22 | 55 | 0.89 | 3 | 48.7 | 6.5 | 51.0 | NA | 0.0 |
| di Minno et al63 | IFX, ETN, ADA | OP | 96 | 270 | 0.88 | 2 | 51.7 | 9.2 | 45.9 | NA | 0.0 |
| Eder et al64 | IFX, ETN, ADA, GOL | OP | 48 | 95 | 0.75 | 2 | 45.7 | 11.8 | 67.9 | NA | 9.6 |
| Gladman et al66 | ADA | RCT | 48 | 285 | 0.61 | 3 | NA | NA | NA | NA | NA |
| Gladman et al65 | ADA | RCT | 24 | 144 | 0.90 | 3 | 47.8 | 9.9 | 43.7 | NA | 0.0 |
| Glintborg et al67 | IFX, ADA, ETN | OR | 24 | 746 | 0.76 | 4 | 47.0† | 5.0† | 52.0 | NA | 0.0 |
| Gratacos et al68 | IFX | OP | 38 | 69 | 0.85 | 2 | 42.5 | 8.0 | 60.8 | NA | 0.0 |
| Iannone et al69 | IFX, ETN, ADA | OR | NA | 135 | 0.86 | 4 | 53.2‡ | 10.0‡ | 49.6 | NA | 0.0 |
| Iervolino et al70 | IFX, ETN, ADA | OP | 12 | 136 | 0.90 | 2 | 45.6 | 5.2 | 58.4 | NA | 0.0 |
| Karanikolas et al71 | ADA | RCT | 48 | 113 | 0.88 | 3 | 46.3 | 7.9 | 55.7 | 23.0 | 0.0 |
| Kavanaugh et al72 | IFX | RCT | 54 | 100 | 0.67 | 3 | 47.1 | 8.4 | 29.0 | NA | 0.0 |
| Kavanaugh et al73 | GOL | RCT | 24 | 292 | 0.65 | 3 | 46.9 | 7.4 | 40.0 | NA | 0.0 |
| Kristensen et al74 | IFX, ETN, ADA | OP | 48 | 261 | 0.70 | 2 | 47.3 | 8.4 | 50.5 | NA | 0.0 |
| Marotta et al75 | ADA | OP | 12 | 24 | 0.53 | 3 | NA | NA | NA | NA | NA |
| Mease et al76 | ADA | RCT | 12 | 151 | 0.68 | 3 | 48.6 | 9.8 | 43.7 | NA | 0.0 |
| Morales-Lara et al43 | IFX | OP | 48 | 16 | 0.50 | 2 | NA | NA | NA | NA | NA |
| Ramirez et al77 | IFX, ETN, ADA | OP | 24 | 103 | 0.78 | 2 | 49.0† | 12.0† | 47.6 | 23.3 | 0.0 |
| Saber et al78 | IFX, ETN, ADA | OP | 12 | 152 | 0.73 | 2 | 45.0† | 8.0† | 52.3 | NA | 0.0 |
| Spadaro et al79 | ETN | OP | NA | 82 | 0.56 | 3 | 51.8 | 9.1 | 42.6 | NA | NA |
| Van den Bosch et al80 | ADA | OP | 12 | 442 | 0.76 | 2 | 47.8 | 10.6 | 50.0 | 23.3 | 14.9 |
| Wagner et al81 | GOL | RCT | 14 | 74 | 0.80 | 3 | 48.5 | NA | 36.0 | NA | 0.0 |
*Data are expressed in mean (years).
†Data are expressed in medians.
‡Data were calculated in the review.
ADA, adalimumab; DD, disease duration; ETN, etanercept; GOL, golimumab; IFX, infliximab; LE, level of evidence; N, number of patients; NA, not available; OP, observational prospective; OR, observational retrospective; Q, quality; RCT, randomised clinical trial.
Demographic and environmental factors
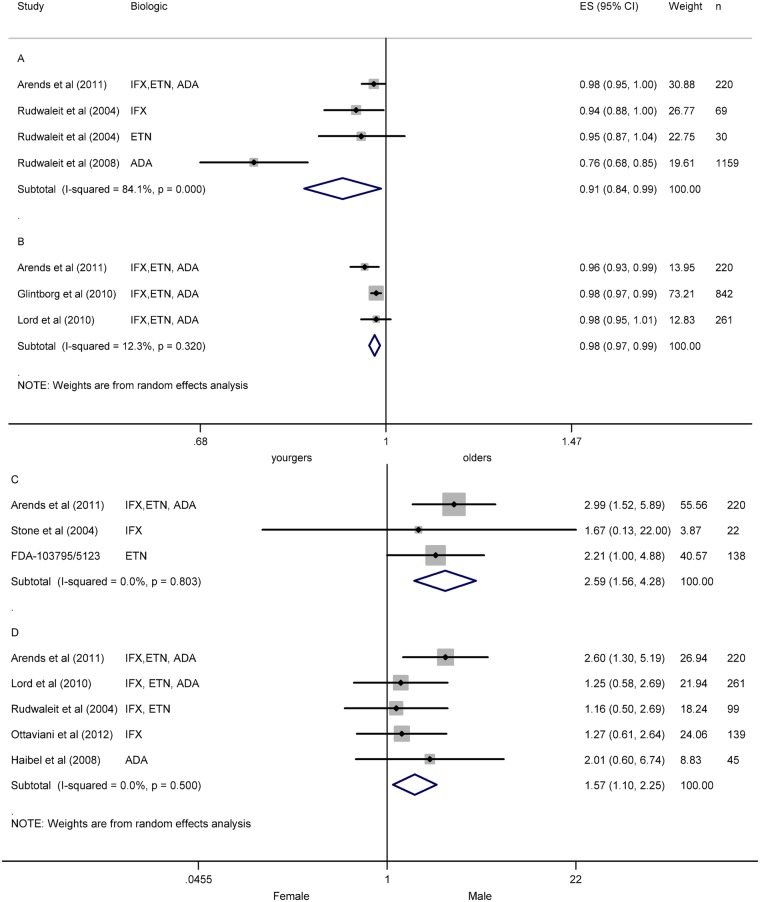
Thirteen studies included data about a demographic or environmental factor as predictor of response in AS.25 26 32 35 39 40 46 49 50–52 56 57 65 Age was analysed in 12 studies.25 26 32 35 40 46 49–52 56 65 Individual results showed better ASAS20,25 26 ASAS4026 35 50 and BASDAI50 responses in younger patient.26 35 40 46 50–52 Meta-analyses of age and BASDAI50 at 12 weeks were performed using data from two studies26 51 and from subgroups of one study,52 as well as with 24 weeks’ data from three studies.26 34 40 Analyses demonstrated a resulting OR (CI 95%) of 0.91 (0.84 to 0.99) with I2 of 84.1% (figure 1A) and no risk of publication bias (Egger test p=0.178), and 0.98 (0.97 to 0.99) with I2 12.3% (figure 1B) and no risk of publication bias (p=0.698) at 12 and 24 weeks, respectively. No factors were identified as a source of heterogeneity.
Figure 1.
Meta-analysis of demographic factors as predictor of response in ankylosing spondylitis (AS). (A) Meta-analysis of age and BASDAI50 at week 12 in AS. (B) Meta-analysis of age and BASDAI50 at week 24 in AS. (C) Meta-analysis of gender and ASAS20 in AS. (D) Meta-analysis of gender and BASDAI50 in AS. ES: effect size (OR).
Gender was analysed in 10 studies.25 26 32 35 39 40 46 49 52 56 Results of individual studies showed better ASAS20,25 26 ASAS4026 and ASDAS responses in men.32 49 Meta-analysis of gender and ASAS20 in three studies showed an OR of 2.58 (1.56 to 4.28) with an I2 of 0.0% (figure 1C), and no risk of publication bias (p=0.854).25 26 56 Individual studies that analysed BASDAI presented contradictory results.26 35 40 52 56 Meta-analysis of gender and BASDAI50 including five studies showed an OR of 1.57 (1.10 to 2.25) with an I2 of 0.0% (figure 1D), and no risk of publication bias (p=0.085).26 35 40 46 52 In one study, high body mass index (BMI) was related with poor BASDAI.46 Smoking was analysed in one study with not significant results.40
In PsA, eight studies analysed demographic factors as potential predictors of response.63 64 67–70 78 81 Five studies included data about age.64 67–70 Only one study showed significant reverse association between age and minimal disease activity (MDA) response.70 Eight studies included data about gender.63 64 67–70 78 81 Men showed better response than women in five studies.63 67 69 78 81 One study showed a negative association of BMI with MDA response.63 Whereas another study showed no association between BMI and DAS28 remission.69
Clinical factors
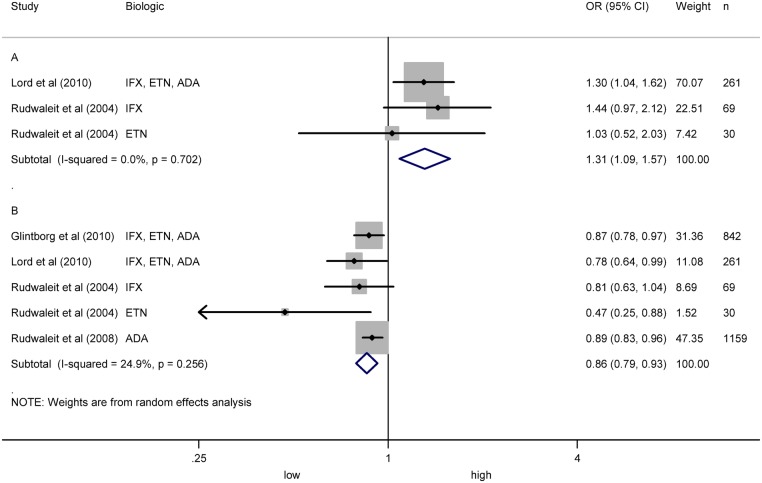
Twenty-one articles included data about clinical factors as predictors of response in AS.25 26 29 30 32 34 35 39 40–42 44 46 48–53 55 56 Five studies included data on BASDAI baseline.26 40 52 55 56 Individual results showed that higher baseline BASDAI predicts better BASDAI5040 52 and ASDAS,55 but not ASAS20 response.56 Meta-analysis of baseline BASDAI and BASDAI50 in one study40 and subgroups of another study52 showed an OR of 1.31 (1.09 to 1.57) with I2 of 0.0%, and no risk of publication bias (p=0.673) (figure 2A). Eight studies analysed baseline BASFI.26 30 34 40 51 52 55 56 Individual results showed that higher baseline BASFI predicts poor BASDAI50 response,34 40 51 52 but not ASAS20 response.26 30 56 A meta-analysis including four studies showed an OR of 0.86 (0.79 to 0.93) with I2 of 24.9% (figure 2B) and risk of publication bias (p=0.004).34 40 51 52
Figure 2.
Meta-analysis of BASDAI baseline and BASFI baseline as predictors of response in ankylosing spondylitis (AS). (A) Meta-analysis of BASDAI baseline and BASDAI50 in AS. (B) Meta-analysis of BASFI baseline and BASDAI50 in AS.
Use of concomitant DMARDs was analysed in seven studies,25 39 40 41 44 48 56 with only one reporting significant results.40 Meta-analysis of concomitant DMARD and ASAS20 including four studies showed an OR of 1.47 (0.81 to 266) with I2 of 55.5%, and no risk of publication bias (p=0.471).25 44 48 56 Sensitivity analysis was performed identifying one study as a possible source of heterogeneity.48 This study was removed from the meta-analysis showing an OR of 1.11 (0.52 to 2.11) with I2 of 0.0%. Concomitant methotrexate (MTX) was analysed in five studies.25 39 40 44 48 One study showed significant association with BASDAI50,40 and another with BASDAI50, ASAS20 and ASAS50 responses.48 Meta-analysis of concomitant MTX and ASAS20 including three studies showed an OR of 1.62 (0.74 to 3.54) with I2 of 72.2%, and no risk of bias (p=0.115).25 44 48 No factor was identified as a source of heterogeneity. Other concomitant drugs such as sulfasalazine,25 non-steroidal anti-inflammatory drugs 40 56 or corticosteroids25 40 were not associated with response.
Disease duration was analysed in six studies with contradictory results.25 26 35 40 46 52 Meta-analysis of disease duration and BASDAI50 including one study40 and subgroups of another study52 showed an OR of 0.96 (0.91 to 1.02) with I2 of 63.6%, and no risk of publication bias (p=0.118). No factor was identified as a source of heterogeneity.
Seven studies included data about peripheral arthritis and obtained contradictory results.26 29 32 35 39 42 52 Meta-analysis of peripheral arthritis and ASAS40 in three studies showed an OR of 0.94 (0.74 to 1.19) with an I2 of 79.2%, and no risk of publication bias (p=0.327).29 32 35 Meta-analysis of peripheral arthritis and BASDAI50 in five studies26 29 32 35 42 and subgroups of another study52 showed an OR of 1.13 (0.64 to 1.97) with an I2 of 70.8%, and no risk of publication bias (p=0.780). No factor was identified as a source of heterogeneity. Three studies analysed enthesitis and BASDAI50 and showed an OR of 0.92 (0.84 to 101) with an I2 of 0.0%, and no risk of publication bias (p=0.378).29 52 Extra-articular manifestations such as uveitis, psoriasis or inflammatory bowel disease (IBD) did not present an association with response.25 29 One study that analysed baseline MRI scores showed association with BASDAI50.53 Syndesmophytes also showed association with poor response.55
Sixteen articles analysed several clinical factors in PsA.4 63 64 66–74 76 78–80 Six studies looked at HAQ baseline and obtained contradictory results.64 68–70 78 80 Other measures such as joint count, VAS pain, VAS global or DAS28 baseline also returned with variable results.63 64 70 Thirteen articles analysed concomitant DMARDs as predictor of response.4 64 66 67 69–74 76 79 80 No significant results were reported regardless of the type of concomitant DMARD, including MTX. One study showed better response with concomitant MTX than monotherapy.67 In four studies, meta-analysis of concomitant MTX and ACR20 showed an OR of 1.18 (0.92 to 1.50) with an I2 of 55.1%, and no publication bias (p=0.092).4 66 67 76 No factor was identified as a source of heterogeneity. In three studies, meta-analysis of concomitant MTX and ACR50 produced an OR of 1.23 (0.82 to 1.83) with an I2 of 0.0%, and no risk of publication bias (p=0.782).4 66 76 In three studies, meta-analysis of concomitant MTX and ACR70 presented an OR of 0.70 (0.50 to 1.25) with an I2 of 0.0%, and no risk of publication bias (p=0.144).4 66 76 Other DMARDs such as cyclosporine71 or sulfasalazine80 showed a better response in a combined group than in TNF antagonists monotherapy. Other variables such as large joint involvement,68 80 axial involvement,68 dactylitis,64 70 erosive arthritis68 or disease duration showed contradictory or not significant results.64 68 69
Serological factors
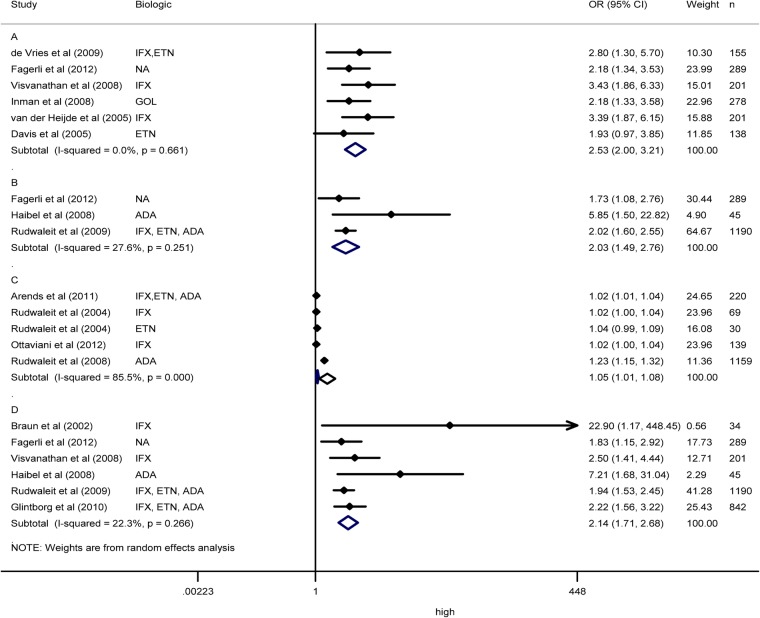
Twenty four articles reported serological factors as predictors of response to TNF antagonists in AS.26–28 30 31 33–35 37–39 41 45–47 49–52 55 56 58–60 Individual results showed better response in patients with high levels of C reactive protein (CRP) in 22 articles.26 28 30 31 33 34 35 37 39 41 45–47 49–52 55 56 58–60 Meta-analysis of CRP and ASAS20 in six articles showed an OR of 2.53 (2.00 to 3.21) with an I2 of 0.0% (figure 3A), and risk of publication bias (p=0.015).30 31 33 37 58 59 Meta-analysis of CRP and ASAS40 in three articles showed an OR of 2.03 (1.49 to 2.76) with an I2 of 27.6% (figure 3B), and no risk of publication bias (p=0.563).33 35 50 Meta-analysis of CRP and BASDAI50 in three articles,26 46 51 and subgroups of another study52 showed an OR of 1.05 (1.01 to 1.08) with an I2 of 85.5% (figure 3C), and risk of publication bias (p=0.008). No factor was identified as a source of heterogeneity. Sensitivity analysis showed one study as a source of heterogeneity, and when this study was removed from the meta-analysis, the OR was of 1.02 (1.01 to 1.03) with an I2 of 0.0%.51 Meta-analysis of dichotomous CRP and BASDAI50 in six articles showed an OR of 2.14 (1.71 to 2.68) with an I2 of 22.4% (figure 3D), and no risk of publication bias (p=0.267).28 33–35 50 59 High levels of serum amyloid A presented an association with better response in one study.31 Erythrocyte sedimentation rate (ESR) showed contradictory results in two studies.26 31 High levels of interleukin (IL)-6 at baseline were related with ASAS but not with BASDAI50 response.47 59 60 Other biomarkers such as matrix metalloproteinase-3 (MMP-3), osteocalcin, insulin, leptin, tissue inhibitor of metalloproteinases 1, apolipoprotein CIII, IgM, N-terminal propeptide of type 1collagen (P1NP), deoxypyridinoline and vascular endothelial growth factor were not consistently associated with response.27 47 59 60
Figure 3.
Meta-analysis of C reactive protein (CRP) as predictor of response in ankylosing spondylitis (AS). (A) Meta-analysis of dichotomous CRP and ASAS20 in AS. (B) Meta-analysis of dichotomous CRP and ASAS40 in AS. (C) Meta-analysis of continuous CRP and BASDAI in AS. (D) Meta-analysis of dichotomous CRP and BASDAI50 in AS. NA: not available.
Twelve studies analysed serological factors as predictor of response in PsA.61 62 64 67–70 75 77 80 81 Nine articles included CRP as a predictor of response, and presented significant association with ACR and MDA response, but this was contradictory with EULAR response.62 63 67–70 75 77 80 No significant results were observed in four studies that analysed ESR.64 68 69 70 In two studies, MMP-3 levels have contradictory results.61 81 Elevated baseline C3 complement levels showed poor association with response in one study.62 Other biomarkers such as adiponectin, ENRAGE (S100A12), IgA, IL-16, insulin and serum glutamic oxaloacetic transaminase were associated with EULAR response but not with ACR20. In contrast, pyridinoline showed association with ACR20 response but not with EULAR response.81
Genetic factors
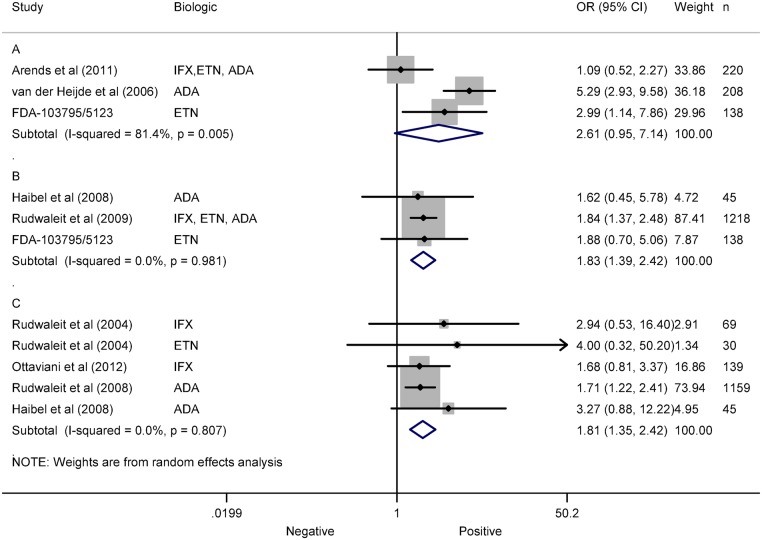
Twelve articles analysed genetic factors as predictors of response to TNF antagonists in AS.1 25 26 32 35 43 46,50–52 54 57 Human leucocyte antigen B27 (HLA-B27) was investigated in nine articles with contradictory results.1 25 26 32 35 46 50–52 Meta-analysis of HLA-B27 and ASAS20 in three studies showed an OR of 2.81 (0.95 to 7.16) with an I2 of 81.5% (figure 4A), and no risk of publication bias (p=0.075).1 25 26 No factor was identified as a source of heterogeneity. Meta-analysis of HLA-B27 and ASAS40 in three studies showed an OR of 1.83 (1.39 to 2.42) with an I2 of 0.0% (figure 1B), and no risk of publication bias (p=0.628).25 35 50 Meta-analysis of HLA-B27 and BASDAI50 in three studies,35 46 51 and subgroups of other study,52 showed an OR of 1.81 (1.35 to 2.42) with an I2 of 0.0% (figure 1C), and no risk of publication bias (p=0.074). No association was shown between −308 TNF gene polymorphism and BASDAI response.54 57 Association was reported of the rs396991 Fc γ-receptor (FCGR) 3A polymorphism with BASDAI50 response.43
Figure 4.
Meta-analysis of human leucocyte antigen B27 (HLAB27) as predictor of response in ankylosing spondylitis (AS). (A) Meta-analysis of HLAB27 and ASAS20 in AS. (B) Meta-analysis of HLAB27 and ASAS40 in AS. (C) Meta-analysis of HLAB27 and BASDAI50 in AS.
Two studies analysed potential genetic predictors of response in PsA.43 77 FCGR3A was reported not to be associated with response to all TNF antagonists in two studies.43 77 However, significant results were observed in a subanalysis of etanercept, but not monoclonal antibodies.77
Discussion
Our review showed that age, gender, baseline BASDAI, baseline BASFI, CRP and HLA-B27 predicts response to TNF antagonists in patients with AS. In contrast, robust predictors of response in PsA were not identified.
In RA, observational studies have suggested that smokers have a poorer response to TNF antagonists than ex-smokers or never smokers.16 82 Higher HAQ baseline has also been related to poor response.13 14 16 82 Other possible predictors of remission with TNF antagonists such as age or gender have been proposed.13 15 82 Better response in younger patients and poor clinical response in women in our meta-analysis of AS was previously reported in patients with RA treated with TNF antagonists.15 17 Studies in PsA also suggest poor response in women, but this could not be confirmed in our meta-analysis.
High BASDAI and high CRP levels predict better response in AS. This could indicate that a subgroup of patients with higher baseline activity may have more benefit from treatment with TNF antagonists. In contrast, BASFI baseline levels are inversely related to response, possibly due to the fact that high BASFI is related in part with established disease and radiological damage. In-line with this, syndesmophytes have also been related with poor response.55 HAQ was also related with poor response in RA and perhaps PsA, as suggested by the individual articles in our review.13 14
In AS and PsA, data from clinical trials have suggested that use of concomitant DMARD does not add benefit to the treatment with TNF antagonists in monotherapy.4 72 73 This is supported by our meta-analysis. Nevertheless, it is reported that the use of concomitant DMARDs decreases the development of antidrug antibodies, and this may be reflected by a lower rate of discontinuation of the biological for any cause.83
Positive HLA-B27 predicts better response to TNF antagonists in patients with AS. TNF is associated with activation of the HLA-B27promoter, and TNF has a pivotal role in the inflammatory component of spondyloarthritis.84 This is consistent with findings from animal model studies, in which a blockade of TNF is related with prevention of IBD and enthesitis in HLA-B27 transgenic rats.85 86 Several other biomarkers of inflammation were found to be related to TNF antagonist response in AS and PsA, but only in a small number of observations. This should be confirmed in subsequent studies.
The principal limitation of the meta-analyses was the variance in the design of studies included in the analysis (clinical trials, and prospective and retrospective observational studies). Furthermore, none of the clinical trials were designed to test the studied association and, thus, they were somehow similar to an observational prospective study regarding risk of bias. In observational studies there is a potential for bias from unmeasured confounding. There is some disagreement on whether meta-analyses should be restricted to include only randomised clinical trials. However, observational studies often represent the best available evidence. Observational studies are thought to over-estimate treatment or exposure effects. Nevertheless, meta-analyses of observational studies continue to be valuable and are commonly used for assessing efficacy and effectiveness, and are increasingly being published in the scientific literature.87 Our review is of predictor factors of response, but not of efficacy. Although the study design is important, there are many other factors influencing the reporting of predictors. The validated Hayden checklist assesses how each study meets the research question (not related to efficacy). All RCTs were of efficacy and predictive variables were not the primary variables. The use of random effects computing summary OR may have potentially accounted for this drawback. Also, to minimise this issue, our analysis of heterogeneity includes not only quality of data but design and level of evidence of the studies. Heterogeneity may help to point out factors that influence the results of the outcome that were not observable in individual trials.88 89 Our statistics included analysis of heterogeneity, risk of bias and quality of data with stringent predefined criteria. The quantitative scales are main tools for assessing risk of bias. The Hayden scale in our study is appropriate because it allows for evaluation of the risk of bias as a relevant variable to identify causes of heterogeneity. Sensitivity analysis was carried out by stratification of meta-analyses by variable causing heterogeneity. Although OR is not the best estimate of association, we used OR because it is readily estimated from the different studies. The review identifies several possible predictors in PsA. However, no conclusive predictors were identified due to the limited number of studies and the heterogeneity of response measures. Also, it is not possible to know whether CRP quantification was carried out using similar or different techniques, and meta-analyses of dichotomous CRP included different cut-offs. Finally, although the findings of some meta-analyses should be interpreted with caution because of the risk of publication bias, our study has several strengths including good consistency of results and inclusion of approximately 60% of studies of high quality.
In conclusion, younger, male sex, high baseline BASDAI, low baseline BASFI, high CRP baseline and positive HLA-B27 predict individually better response in AS. In contrast, no conclusive predictors of PsA are identified.
Footnotes
Contributors: JRM was involved in the data collection, interpretation of data, drafting the article, literature search and selection papers for inclusion. AS was involved in the selection papers for inclusion. ES was involved in the selection papers for inclusion. AM was involved in the study design, interpretation of data, drafting the article and revising it critically for important intellectual content. JJG-R was involved in the conception and study design, interpretation of data, drafting the article and revising it critically for important intellectual content. All authors gave final approval of the version to be published.
Competing interests: JJG-R is on the Advisory Boards of Abbvie, BMS, Pfizer, Roche, MSD and UCB SA; has received lecture fees from Abbvie, BMS, Jansen and Jansen, MSD, Pfizer, Roche and UCB; and has received research grants from Roche, Pfizer, MSD and UCB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.van der Heijde D, Kivitz A, Schiff MH et al. . Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136–46. 10.1002/art.21913 [DOI] [PubMed] [Google Scholar]
- 2.Mease PJ, Goffe BS, Metz J et al. . Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385–90. 10.1016/S0140-6736(00)02530-7 [DOI] [PubMed] [Google Scholar]
- 3.Brandt J, Khariouzov A, Listing J et al. . Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003;48:1667–75. 10.1002/art.11017 [DOI] [PubMed] [Google Scholar]
- 4.Antoni C, Krueger GG, de Vlam K et al. . Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7. 10.1136/ard.2004.032268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landewe R, Braun J, Deodhar A et al. . Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mease PJ, Fleischmann R, Deodhar AA et al. . Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. 10.1136/annrheumdis-2013-203696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavanaugh A, McInnes I, Mease P et al. . Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. 10.1002/art.24403 [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Reino JJ, Carmona L, Valverde VR et al. . Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003;48:2122–7. 10.1002/art.11137 [DOI] [PubMed] [Google Scholar]
- 9.Greenberg JD, Reed G, Kremer JM et al. . Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis 2010;69:380–6. 10.1136/ard.2008.089276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Sola MJ, Torre-Cisneros J, Perez-Zafrilla B et al. . Infections in patients treated with tumor necrosis factor antagonists: incidence, etiology and mortality in the BIOBADASER registry. Med Clin (Barc) 2011;137:533–40. 10.1016/j.medcli.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb A, Menter A, Mendelsohn A et al. . Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373:633–40. 10.1016/S0140-6736(09)60140-9 [DOI] [PubMed] [Google Scholar]
- 12.Schett G, Wollenhaupt J, Papp K et al. . Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2012;64:3156–67. 10.1002/art.34627 [DOI] [PubMed] [Google Scholar]
- 13.Hyrich KL, Watson KD, Silman AJ et al. . Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology 2006;45:1558–65. 10.1093/rheumatology/kel149 [DOI] [PubMed] [Google Scholar]
- 14.Kristensen LE, Kapetanovic MC, Gulfe A et al. . Predictors of response to anti-TNF therapy according to ACR and EULAR criteria in patients with established RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 2008;47:495–9. 10.1093/rheumatology/ken002 [DOI] [PubMed] [Google Scholar]
- 15.Mancarella L, Bobbio-Pallavicini F, Ceccarelli F et al. ; GISEA group. Good clinical response, remission, and predictors of remission in rheumatoid arthritis patients treated with tumor necrosis factor-alpha blockers: the GISEA study. J Rheumatol 2007;34:1670–3. [PubMed] [Google Scholar]
- 16.Mattey DL, Brownfield A, Dawes PT. Relationship between pack-year history of smoking and response to tumor necrosis factor antagonists in patients with rheumatoid arthritis. J Rheumatol 2009;36:1180–7. 10.3899/jrheum.081096 [DOI] [PubMed] [Google Scholar]
- 17.Radovits BJ, Kievit W, Fransen J et al. . Influence of age on the outcome of antitumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis 2009;68:1470–3. 10.1136/ard.2008.094730 [DOI] [PubMed] [Google Scholar]
- 18.Maneiro RJ, Salgado E, Carmona L et al. . Rheumatoid factor as predictor of response to abatacept, rituximab and tocilizumab in rheumatoid arthritis: Systematic review and meta-analysis. Semin Arthritis Rheum 2013;43:9–17. 10.1016/j.semarthrit.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. 10.7326/0003-4819-144-6-200603210-00010 [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M et al. . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Health & Human Services. Food & Drug Administration. 103795/5123, Etanercept in Ankylosing Spondylitis. Medical Review 2003. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/103795–5123_Enbrel_medr.pdf
- 26.Arends S, Brouwer E, van der Veer E et al. . Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 2011;13:R94 10.1186/ar3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arends S, van der Veer E, Groen H et al. . Serum MMP-3 level as a biomarker for monitoring and predicting response to etanercept treatment in ankylosing spondylitis. J Rheumatol 2011;38:1644–50. 10.3899/jrheum.101128 [DOI] [PubMed] [Google Scholar]
- 28.Braun J, Brandt J, Listing J et al. . Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93. 10.1016/S0140-6736(02)08215-6 [DOI] [PubMed] [Google Scholar]
- 29.Braun J, Rudwaleit M, Kary S et al. . Clinical manifestations and responsiveness to adalimumab are similar in patients with ankylosing spondylitis with and without concomitant psoriasis. Rheumatology (Oxford) 2010;49:1578–89. 10.1093/rheumatology/keq129 [DOI] [PubMed] [Google Scholar]
- 30.Davis JC, van der Heijde D, Dougados M et al. . Baseline factors that influence ASAS 20 response in patients with ankylosing spondylitis treated with etanercept. J Rheumatol 2005;32:1751–4. [PubMed] [Google Scholar]
- 31.de Vries MK, van Eijk IC, van der Horst-Bruinsma IE et al. . Erythrocyte sedimentation rate, C-reactive protein level, and serum amyloid a protein for patient selection and monitoring of anti-tumor necrosis factor treatment in ankylosing spondylitis. Arthritis Rheum 2009;61:1484–90. 10.1002/art.24838 [DOI] [PubMed] [Google Scholar]
- 32.Fagerli KM, Lie E, Heiberg MS et al. . Predictors of ASDAS major improvement in patients with ankylosing spondylitis receiving their first TNF inhibitor. Results rrom a longitudinal observational study. Arthritis Rheum 2011;63:S204–5. [Google Scholar]
- 33.Fagerli KM, Lie E, van der Heijde D et al. . Selecting patients with ankylosing spondylitis for TNF inhibitor therapy: comparison of ASDAS and BASDAI eligibility criteria. Rheumatology 2012;51:1479–83. 10.1093/rheumatology/kes057 [DOI] [PubMed] [Google Scholar]
- 34.Glintborg B, Ostergaard M, Krogh NS et al. . Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 2010;69:2002–8. 10.1136/ard.2009.124446 [DOI] [PubMed] [Google Scholar]
- 35.Haibel H, Rudwaleit M, Listing J et al. . Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91. 10.1002/art.23606 [DOI] [PubMed] [Google Scholar]
- 36.Huang F, Zhu J, Zhang L et al. . Response to one infusion predicts subsequent improvement as well as the rate of relapse of ankylosing spondylitis infused with three pulses of infliximab. Clin Rheumatol 2007;26:920–6. 10.1007/s10067-006-0434-8 [DOI] [PubMed] [Google Scholar]
- 37.Inman RD, Davis JC Jr, Heijde D et al. . Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402–12. 10.1002/art.23969 [DOI] [PubMed] [Google Scholar]
- 38.Kim TH, Stone M, Payne U et al. . Cartilage biomarkers in ankylosing spondylitis: relationship to clinical variables and treatment response. Arthritis Rheum 2005;52:885–91. 10.1002/art.20870 [DOI] [PubMed] [Google Scholar]
- 39.Kristensen LE, Karlsson JA, Englund M et al. . Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: an observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res (Hoboken) 2010;62:1362–9. 10.1002/acr.20258 [DOI] [PubMed] [Google Scholar]
- 40.Lord PA, Farragher TM, Lunt M et al. . Predictors of response to anti-TNF therapy in ankylosing spondylitis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2010;49:563–70. 10.1093/rheumatology/kep422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luc M, Gossec L, Ruyssen-Witrand A et al. . C-reactive protein predicts tumor necrosis factor-alpha blocker retention rate in axial ankylosing spondylitis. J Rheumatol 2007;34:2078–81. [PubMed] [Google Scholar]
- 42.Maria Lizzio M, Peluso G, Zoli A et al. . Analysis of response to infliximab in ankylosing spondylitis according to the axial and/or peripheral involvement: autoantibodies and drop outs are more frequent in the peripheral subset. Ann Rheum Dis 2007;66:427–8. 10.1136/ard.2006.065052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Lara MJ, Conesa-Zamora P, Garcia-Simon MS et al. . Association between the FCGR3A V158F polymorphism and the clinical response to infliximab in rheumatoid arthritis and spondyloarthritis patients. Scand J Rheumatol 2010;39:518–20. 10.3109/03009741003781969 [DOI] [PubMed] [Google Scholar]
- 44.Mulleman D, Lauferon F, Wendling D et al. . Infliximab in ankylosing spondylitis: alone or in combination with methotrexate? A pharmacokinetic comparative study. Arthritis Res Ther 2011;13:R82 10.1186/ar3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarro-Compan V, Ariza-Ariza R, Mondejar-Garcia R et al. . Metalloproteinase-3 (MMP-3) is a predictor for anti-TNF-alpha response in patients with ankylosing spondylitis (AS). Clin Exp Rheumatol 2012;30:638. [Google Scholar]
- 46.Ottaviani S, Allanore Y, Tubach F et al. . Body mass index influences the response to infliximab in ankylosing spondylitis. Arthritis Res Ther 2012;14:R115 10.1186/ar3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedersen SJ, Sorensen IJ, Garnero P et al. . ASDAS, BASDAI and different treatment responses and their relation to biomarkers of inflammation, cartilage and bone turnover in patients with axial spondyloarthritis treated with TNFalpha inhibitors. Ann Rheum Dis 2011;70:1375–81. 10.1136/ard.2010.138883 [DOI] [PubMed] [Google Scholar]
- 48.Perez-Guijo VC, Cravo AR, Castro Mdel C et al. . Increased efficacy of infliximab associated with methotrexate in ankylosing spondylitis. Joint Bone Spine 2007;74:254–8. 10.1016/j.jbspin.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 49.Ramiro S, Machado P, Roque R et al. . Predictive factors of response at 12 weeks in patients with ankylosing spondylitis starting biological therapies—results from the portuguese register—Reuma.Pt. Clin Exp Rheumatol 2012;30:641–2. [Google Scholar]
- 50.Rudwaleit M, Claudepierre P, Wordsworth P et al. . Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 2009;36:801–8. 10.3899/jrheum.081048 [DOI] [PubMed] [Google Scholar]
- 51.Rudwaleit M, Claudepierre P, Wordsworth P et al. . Predictors of good clinical response (BASDAI 50 or ASAS partial remission) in 1,250 patients treated with adalimumab (humira (R)) for active ankylosing spondylitis (AS). Clin Exp Rheumatol 2008;26:744. [Google Scholar]
- 52.Rudwaleit M, Listing J, Brandt J et al. . Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis 2004;63:665–70. 10.1136/ard.2003.016386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudwaleit M, Schwarzlose S, Hilgert ES et al. . MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis 2008;67:1276–81. 10.1136/ard.2007.073098 [DOI] [PubMed] [Google Scholar]
- 54.Seitz M, Wirthmuller U, Moller B et al. . The -308 tumour necrosis factor-alpha gene polymorphism predicts therapeutic response to TNFalpha-blockers in rheumatoid arthritis and spondyloarthritis patients. Rheumatology 2007;46:93–6. 10.1093/rheumatology/kel175 [DOI] [PubMed] [Google Scholar]
- 55.Sieper J, van der Heijde D, Dougados M et al. . Early response to adalimumab predicts long-term remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:700–6. 10.1136/annrheumdis-2011-200358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone MA, Payne U, Pacheco-Tena C et al. . Cytokine correlates of clinical response patterns to infliximab treatment of ankylosing spondylitis. Ann Rheum Dis 2004;63:84–7. 10.1136/ard.2003.006916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong Q, Zhao DB, Bajracharya P et al. . TNF-alpha -857 and -1031 polymorphisms predict good therapeutic response to TNF-alpha blockers in Chinese Han patients with ankylosing spondylitis. Pharmacogenomics 2012;13:1459–67. 10.2217/pgs.12.133 [DOI] [PubMed] [Google Scholar]
- 58.van der Heijde D, Dijkmans B, Geusens P et al. . Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582–91. 10.1002/art.20852 [DOI] [PubMed] [Google Scholar]
- 59.Visvanathan S, Wagner C, Marini JC et al. . Inflammatory biomarkers, disease activity and spinal disease measures in patients with ankylosing spondylitis after treatment with infliximab. Ann Rheum Dis 2008;67:511–17. 10.1136/ard.2007.071605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner C, Visvanathan S, Braun J et al. . Serum markers associated with clinical improvement in patients with ankylosing spondylitis treated with golimumab. Ann Rheum Dis 2012;71:674–80. 10.1136/ard.2010.148890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandran V, Shen H, Pollock R et al. . Soluble biomarkers predict response to anti-tumour necrosis factor (TNF) therapy in psoriatic arthritis (PsA). Arthritis Rheum. 2009; Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 09 Atlanta, GA United States Conference Start: 20101106 Conference End: 20101111. Conference Publication: (var.pagings). 60:1778. [Google Scholar]
- 62.Chimenti MS, Perricone C, Graceffa D et al. . Complement system in psoriatic arthritis: a useful marker in response prediction and monitoring of anti-TNF treatment. Clin Exp Rheumatol 2012;30:23–30. [PubMed] [Google Scholar]
- 63.di Minno MN, Peluso R, Iervolino S et al. . Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res (Hoboken) 2013;65:141–7. 10.1002/acr.21711 [DOI] [PubMed] [Google Scholar]
- 64.Eder L, Chandran V, Schentag CT et al. . Time and predictors of response to tumour necrosis factor-alpha blockers in psoriatic arthritis: an analysis of a longitudinal observational cohort. Rheumatology (Oxford) 2010;49:1361–6. 10.1093/rheumatology/keq091 [DOI] [PubMed] [Google Scholar]
- 65.Gladman DD, Mease PJ, Choy EH et al. . Risk factors for radiographic progression in psoriatic arthritis: subanalysis of the randomized controlled trial ADEPT. Arthritis Res Ther 2010;12:R113 10.1186/ar3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gladman DD, Mease PJ, Ritchlin CT et al. . Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum 2007;56:476–88. 10.1002/art.22379 [DOI] [PubMed] [Google Scholar]
- 67.Glintborg B, Ostergaard M, Dreyer L et al. . Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor alpha therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382–90. 10.1002/art.30117 [DOI] [PubMed] [Google Scholar]
- 68.Gratacos J, Casado E, Real J et al. . Prediction of major clinical response (ACR50) to infliximab in psoriatic arthritis refractory to methotrexate. Ann Rheum Dis 2007;66:493–7. 10.1136/ard.2006.060079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iannone F, Fanizzi R, Scioscia C et al. . Body mass does not affect the remission of psoriatic arthritis patients on anti-TNF-alpha therapy. Scand J Rheumatol 2013;42:41–4. 10.3109/03009742.2012.715186 [DOI] [PubMed] [Google Scholar]
- 70.Iervolino S, Di Minno MN, Peluso R et al. . Predictors of early minimal disease activity in patients with psoriatic arthritis treated with tumor necrosis factor-alpha blockers. J Rheumatol 2012;39:568–73. 10.3899/jrheum.110763 [DOI] [PubMed] [Google Scholar]
- 71.Karanikolas GN, Koukli EM, Katsalira A et al. . Adalimumab or cyclosporine as monotherapy and in combination in severe psoriatic arthritis: results from a prospective 12-month nonrandomized unblinded clinical trial. J Rheumatol 2011;38:2466–74. 10.3899/jrheum.110242 [DOI] [PubMed] [Google Scholar]
- 72.Kavanaugh A, Krueger GG, Beutler A et al. . Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2007;66:498–505. 10.1136/ard.2006.058339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kavanaugh A, van der Heijde D, McInnes IB et al. . Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 2012;64:2504–17. 10.1002/art.34436 [DOI] [PubMed] [Google Scholar]
- 74.Kristensen LE, Gulfe A, Saxne T et al. . Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish Arthritis Treatment Group register. Ann Rheum Dis 2008;67:364–9. 10.1136/ard.2007.073544 [DOI] [PubMed] [Google Scholar]
- 75.Marotta A, Van Kuijk AW, Maksymowych WP et al. . 14-3-3 Eta is a modifiable serum biomarker that marks adalimumab response in psoriatic arthritis. Arthritis Rheum. 2012 October; Conference: Annual Scientific Meeting of the American College of Rheumatology and Association of Rheumatology Health Professionals 2012 Washington, DC United States Conference Start: 20121109 Conference End: 20121114. Conference Publication: (var.pagings). 64:S247. [Google Scholar]
- 76.Mease PJ, Gladman DD, Ritchlin CT et al. . Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. 10.1002/art.21306 [DOI] [PubMed] [Google Scholar]
- 77.Ramirez J, Fernandez-Sueiro JL, Lopez-Mejias R et al. . FCGR2A/CD32A and FCGR3A/CD16A variants and EULAR response to tumor necrosis factor-alpha blockers in psoriatic arthritis: a longitudinal study with 6 months of followup. J Rheumatol 2012;39:1035–41. 10.3899/jrheum.110980 [DOI] [PubMed] [Google Scholar]
- 78.Saber TP, Ng CT, Renard G et al. . Remission in psoriatic arthritis: is it possible and how can it be predicted? Arthritis Res Ther 2010;12:R94 10.1186/ar3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spadaro A, Ceccarelli F, Scrivo R et al. . Life-table analysis of etanercept with or without methotrexate in patients with psoriatic arthritis. Ann Rheum Dis 2008;67:1650–1. 10.1136/ard.2007.085951 [DOI] [PubMed] [Google Scholar]
- 80.Van den Bosch F, Manger B, Goupille P et al. . Effectiveness of adalimumab in treating patients with active psoriatic arthritis and predictors of good clinical responses for arthritis, skin and nail lesions. Ann Rheum Dis 2010;69:394–9. 10.1136/ard.2009.111856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wagner CL, Visvanathan S, Elashoff M et al. . Markers of inflammation and bone remodelling associated with improvement in clinical response measures in psoriatic arthritis patients treated with golimumab. Ann Rheum Dis 2013;72:83–8. 10.1136/annrheumdis-2012-201697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abhishek A, Butt S, Gadsby K et al. . Anti-TNF-alpha agents are less effective for the treatment of rheumatoid arthritis in current smokers. J Clin Rheumatol 2010;16:15–18. 10.1097/RHU.0b013e3181ca4a2a [DOI] [PubMed] [Google Scholar]
- 83.Maneiro JR, Salgado E, Gomez-Reino JJ. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated Inflammatory conditions: systematic review and meta-analysis. JAMA Intern Med 2013;173:1416–28. 10.1001/jamainternmed.2013.7430 [DOI] [PubMed] [Google Scholar]
- 84.Zhao L, Fong Y, Granfors K et al. . Identification of cytokines that might enhance the promoter activity of HLA-B27. J Rheumatol 2008;35:862–8. [PubMed] [Google Scholar]
- 85.Milia AF, Ibba-Manneschi L, Manetti M et al. . Evidence for the prevention of enthesitis in HLA-B27/hbeta(2)m transgenic rats treated with a monoclonal antibody against TNF-alpha. J Cell Mol Med 2011;15:270–9. 10.1111/j.1582-4934.2009.00984.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milia AF, Manetti M, Generini S et al. . TNFalpha blockade prevents the development of inflammatory bowel disease in HLA-B27 transgenic rats. J Cell Mol Med 2009;13:164–76. 10.1111/j.1582-4934.2008.00310.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stroup DF, Berlin JA, Morton SC et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 88.Dickersin K, Min YI, Meinert CL. Factors influencing publication of research results. Follow-up of applications submitted to two institutional review boards. JAMA 1992;267:374–8. 10.1001/jama.1992.03480030052036 [DOI] [PubMed] [Google Scholar]
- 89.Biggerstaff BJ, Tweedie RL. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat Med 1997;16:753–68. [DOI] [PubMed] [Google Scholar]