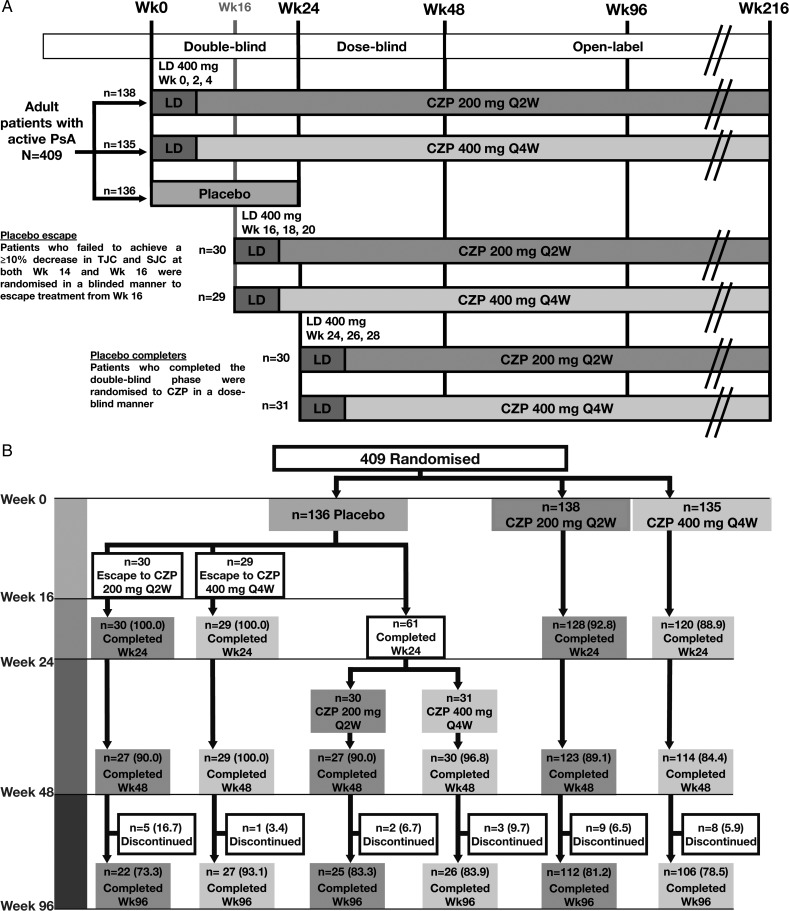
Figure 1.
(A) Trial design and (B) Patient disposition to week 96 of the RAPID-PsA trial, and (C) Kaplan-Meier plot of time to withdrawal for any reason, and due to lack of efficacy or adverse events for patients originally randomised to CZP. Data shown for the randomised set. AE, adverse event; CZP, certolizumab pegol; LD, loading dose; Q2W, every 2 weeks; wk, week; PsA, psoriatic arthritis; SJC, swollen joint count; TJC, tender joint count.