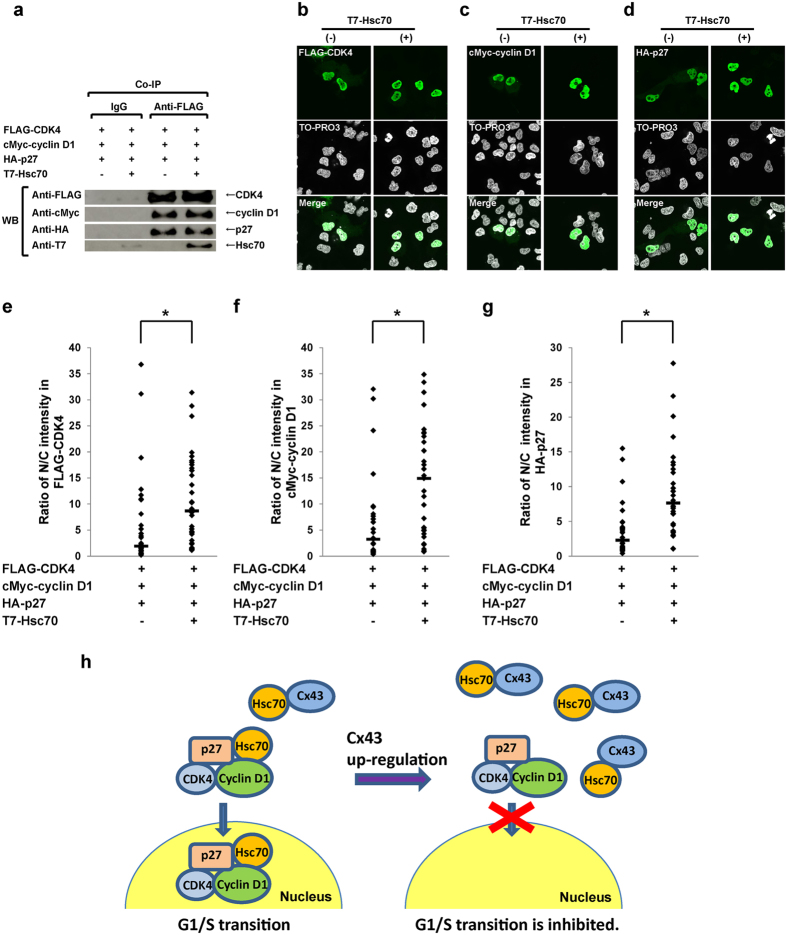
Figure 6. Hsc70 regulates nuclear translocation of cyclin D1-CDK4-p27 complex.
(a) Hsc70 did not affect the assembly of cyclin D1-CDK4-p27 complex. HuH-7 cells were transfected with equivalent plasmids of HA-p27, cMyc-cyclin D1and FLAG-CDK4 with or without T7-Hsc70. Lysates were immunoprecipitated by control IgG or anti-FLAG-tag antibody. The immunocomplexes were analyzed on 12% SDS-PAGE followed by western blotting (WB) using anti-FLAG-tag or anti-HA-tag, and/or anti-cMyc-tag, or anti-T7 tag antibodies. Cropped blots are shown. (b–d) Subcellular localization of the complex components CDK4 (b), cyclin D1 (c) and p27 (d). HuH-7 cells were transfected equivalent plasmids of HA-p27, cMyc-cyclin D1 and FLAG-CDK4 with or without T7-Hsc70. After 48 hr of transfection, immunofluorescence staining with anti-FLAG-tag or anti-cMyc-tag, and/or anti-HA-tag antibodies was carried out, respectively. (e–g) Ratio of nuclear/cytoplasmic (N/C) fluorescence intensities in CDK4 (e), cyclin D1 (f), and p27 (g). Nucleocytoplasmic localization analysis was performed in FLAG-tag, cMyc-tag and HA-tag, respectively. At least 30 transfected HuH-7 cells were examined and quantified for each sample. The data were plotted, and the horizontal lines represent median values. *p < 0.05. (h) A schematic illustration of the mechanism by which Cx43 regulates G1/S transition. Hcs70 interacts with the cyclin D1-CDK4-p27 complex and enhances the nuclear translocation of the complex, leading to G1/S transition (left). When Cx43 is up-regulated (right), the Cx43-Hsc70 interaction prevents the cyclin D1-CDK4-p27 complex enhancement for the nuclear translocation, causing inhibition of G1/S transition.