Abstract
Emodin, a major bioactive extract of several Chinese herbs, has been shown to have a number of biological activities including antiviral, anti-inflammatory, anti-tumor, anti-fibrosis etc. In the present study, we investigated the effects of emodin as an anti-inflammatory agent on lipopolysaccharide (LPS) induced keratitis in Wistar rats. Clinical score, slit-lamp microscope were used to determine corneal inflammatory response. Corneal structure was observed by hematoxylin-eosin staining and transmission electron microscopy. Messenger ribonucleic acid levels of tight junction protein and cytokines were determined by reverse transcription- polymerase chain reaction. The activation of nuclear factor-kappa B (NF-κB) was detected with Western blot. We found that disorganized corneal tissue and cellular structures were observed in keratitis rats and emodin could deduce inflammatory response and improve corneal structure. Pretreated with emodin could up-regulate and down-regulate the mRNA expression of occludin and Interleukin-6. The activation of NF-κB could be inhibited partly after emodin treatment. In conclusion, emodin reduced corneal inflammation in LPS-induced keratitis in Wistar rats due to its capability of inhibition in NF-κB activation.
Keywords: Chinese herb, lipopolysaccharides, nuclear factor-kappa B, inflammation
Introduction
Bacterial keratitis is a common and severe ocular infection and one of the key causes of blindness and visual disability throughout the world [1,2]. Bacterial infections occurred in the cornea after an injury or under other pathological conditions may induce infiltration of inflammatory cells, hamper the transparency of the cornea and even cause permanent vision loss due to scarring and perforation.
Many cytokines and inflammatory mediators’ genes expression increase in corneal tissue during inflammation. The expression of cytokines is regulated by special transcription factors. Nuclear factor-kappa B (NF-κB), a multi-subunit transcription factor which rapidly activates the transcription of various cytokines and chemokines, plays a critical role in the inflammatory response [3]. Numerous studies have investigated the roles of NF-κB in ocular surface disorders [4].
Emodin (1, 3, 8-trihydroxy-6-methyl-anthraquinone) is an anthraquinone and is the major bioactive compound found in several Chinese herbs. Emodin has been shown to have a number of biological activities, such as, anti-microbial, anti-inflammatory, anti-viral, anti-fibrosis and immunosuppressive [5,6]. On molecular level, it acts as a NF-κB inhibitor [7,8]. In the present study, the role of emodin as an anti-inflammatory agent in the lipopolysaccharide (LPS)-induced keratitis of rats was investigated.
Materials and methods
Rat model of LPS-induced keratitis
Wistar rats, 230 to 250 g of weight, were purchased from the Animal Center of Shandong University and housed in the same laboratory conditions. All animals were maintained according to institutional guidelines and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Rats were randomly divided into inflammation, control and treatment group, and were pretreated with nothing, dimethyl sulfoxide and emodin 30 min before LPS challenged respectively. Each rat was anesthetized by intraperitoneal injection of 40 mg/kg pentobarbital sodium. Topical anesthesia was also achieved with one drop of 0.5% proparacaine in the right eye. Then the right eye was wounded with three parallel abrasions using a 26-gauge needle that only penetrated the corneal epithelial cell layer, as described previously [9], and a 10 µl aliquot containing 50 µg LPS was applied to each scarified cornea. Rats were examined at different post-infection periods to ensure that their eyes were similarly infected and to monitor the course of disease.
Evaluation of cornel inflammation
Inflammatory response was evaluated with a slit lamp microscope (Six Six Visual Inc. Suzhou, China) at 1, 6, 12, 24 and 72 h after LPS challenged. Rat eyes were graded by using a scale previously described by Girgis et al. [10]. 0, clear and normal; +1, readily detectable opacity; +2, dense opacity or opacity partially covering the entire corneal surface over pupil; +3, dense opacity covering entire corneal surface over pupil; +4, moderate to dense opacity covering entire corneal surface with corneal erosion. Mean SLE scores were calculated by summation of the scores for each group of rat divided by the total number of rats graded at each time point.
Histological examination
At different points following LPS treatment, 6 eyes randomly selected from each group were enucleated and washed immediately in running water, and then they were rinsed with physiological buffer solution. Then the cornea was excised from the eyeball at the limbus by a routine procedure. Each corneal specimen was divided into two parts. One-half was subjected to histological examination and the other to transmission electron microscopic study. For histological examination, corneas were fixed in 4% paraformaldehyde for 6 h and embedded in paraffin by a routine procedure. Then consecutive 4 μm sections were cut (Leica, Germany) and routinely stained by hematoxylin and eosin for light microscopy examination and evaluation. The histological sections were examined and inflamed cells were count by 3 pathology experts at the same time, and then gave a consistent result for each section.
Transmission electron microscopy
For electron microscopic investigation, the cornea was rinsed with 4°C PBS, and then fixed for 2 h in cold 2.5% glutaraldehyde and then for 2 h in 1% osmic acid (Wako Pure Chemical Industries, Ltd, Japan). After extensively rinsed with PBS, they were dehydrated in a graded series of alcohol and embedded in Epon812 (Heidelberg Ltd, USA). Thin sections were prepared and stained with uranyl acetate and lead citrate. Specimens were observed and photographed with a H-7500 transmission electron microscopy (Hitachi Ltd, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from corneal tissue using Trizol reagent according to manufacturer’s indications. Concentration was detected by measuring the absorbance at 260 nm. Reverse transcription using primers was performed on DNase-treated RNA. After RNase treatment, phenol extraction, and precipitation, the resuspended cDNA was quantified by optical density. β-actin was used as internal standard. The primers used for PCR reactions were 5’-AAGATCCTGACCGAGCGTGG-3’ and 5’-CAGCACTGTGTTGGCATAGAG-3’ for β-actin; 5’-ATACCACCCACAACAGACCAGT-3’ and 5’-GATGAGTTGGATGGTCTTGGT-3’ for Interleukin-6 (IL-6); 5’-GCTCAGGGAATATCCACCTATCA-3’ and 5’-CACAAAGTTTTAACTTCC CAGACG-3’ for Occludin. The primers were designed and synthesized by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). The PCR products were separated by agarose gel electro-phoresis and photographed under ultraviolet light following ethidium bromide staining. Results were quantitated with a gelatine, image analysis system (Kodak System, Japan) using ratios of target gene mRNA to β-actin’s.
Western blot analysis
Nuclear proteins were extracted from frozen corneal tissue according to the manufacturer’s instructions. Protein concentrations were determined and equivalent amounts of the samples (30 μg) were separated on 12% sodium dodecyl sulfate-polyacrylamide gels. Separated proteins were electro-transferred to a nitrocellulose membrane (Millipore, Bedford, USA). Membranes were blocked for 2 h with 5% nonfat milk at room temperature, washed three times with PBS, and then were incubated overnight at 4°C with an antibody of NF-κB p65 (1:400; Neomarkers, USA) primary antibodies. After three washes, membranes were incubated for 2 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibody, then were washed twice with PBS at room temperature. The immune complexes were visualized by using of a Western blotting detection reagent (Kodak System, Japan).
Statistical analysis
For statistical analysis, data are expressed as mean ± standard deviation (SD). An analysis of variance (ANOVA factor) was performed to analyze the relationships within and between variables. A probability value of less than 0.05 was considered significant.
Results
Effect of emodin on corneal manifestations
Typical manifestations of acute keratitis, such as conjunctiva hyperaemia, corneal edema, opacity and infiltration, were observed in LPS-induced keratitis model. Compared with the rats in the inflammation group, there was not obvious changes of corneal manifestations in rats of the control group, while the signs, symptoms and the slit lamp examination scores of the corneas significantly improved in emodin pretreated rats before LPS challenged (Figures 1 and 2).
Figure 1.
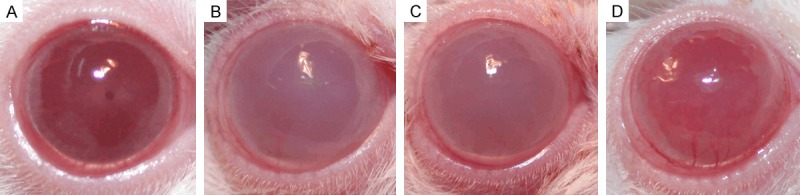
Clinical manifestations of rat’s cornea (6 h). A: Transparent cornea in normal rat; B and C: Dense opacity of cornea in the inflammation and the control group; D: Moderate opacity of cornea in the treatment group.
Figure 2.
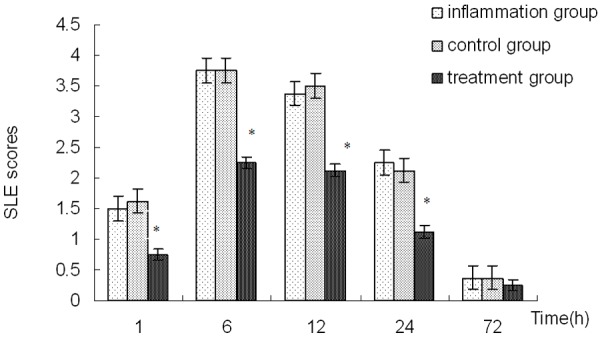
Slit lamp examination scores for rats’ corneas. *Indicates a significant difference from the inflammation group at P<0.01.
Effect of emodin on inflammatory cell invasion
Histochemical analyses of the sections indicated that an obvious infiltration of inflammatory cells was observed in corneas of the inflammation group. The infiltration of inflammatory cells in corneas of the inflammation group (Figure 3A). And the similar situation was presented in the control group (Figure 3B). Most of the inflammatory cells were polymorpho-nuclear leukocytes and a few were lymphocytes or monocytes (Figure 3A and 3B). Inflammatory cells were decreased in corneas of the emodin pretreated group (Figure 3C).
Figure 3.
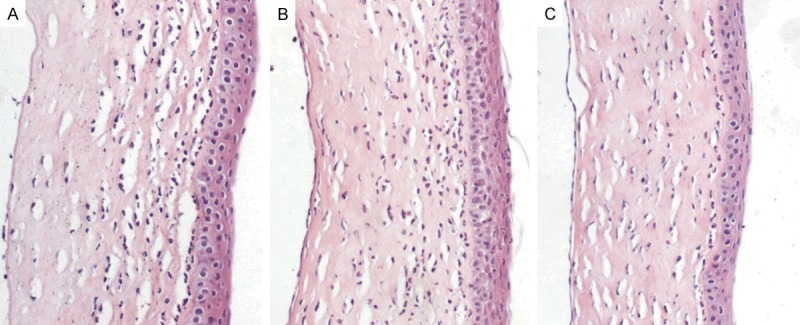
H&E staining of rats corneal histological sections (200×). A large number of inflamed cells were present in sections of the control group (A) and the inflammation group (B); cells decreased considerably after emodin pre-treatment (C).
Effects of emodin on corneal structure
Normal rat cornea had a five-layer structure and each layer arranged clearly. By transmission electron microscopy, the carpet of corneal cellular microvilli, the cellular borders, intercellular junctions and cellular structure were regular and complete. After challenged with LPS, corneal structures were disorganized: unclear structure with loose and fractured layers, irregular arrangement of the collagen fibers and destruction of intercellular junction and extracellular structure. There was no obvious difference in the number of the inflammatory cells between the control group and inflammation group rats, while pretreated with emodin could lighten these damages.
Effect of emodin on tight junction proteins
The expression of occludin, one of the tight junction proteins was detected with RT-PCR analysis. The occludin mRNA were decreased in the inflammation group (P<0.01), and there was no difference between the control and inflammation group (P>0.05). Compared with inflammation group, the expression of occludin in the group pretreated with emodin was significantly up-regulated at 1, 6, 12, and 24 h after LPS administration (Figure 4).
Figure 4.
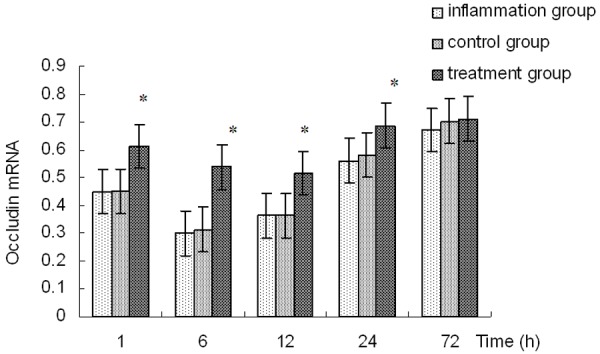
Occludin mRNA expressions in corneas. *Indicates a significant difference from the inflammation group at P<0.01.
Effect of Emodin on cytokine expression
RT-PCR assays were used to test the effects of emodin on cytokine produced in rats’ corneas. IL-6 mRNA expression levels were significantly increased after LPS administration. Significantly decreased levels of IL-6 mRNA expression was observed in corneas of treatment group than those of the inflammation group at 1, 6, 12, and 24 h after LPS administration, and the difference was significant (P<0.01). The expression of IL-6 mRNA was not different between the control group and the inflammation group (P>0.05) (Figure 5).
Figure 5.
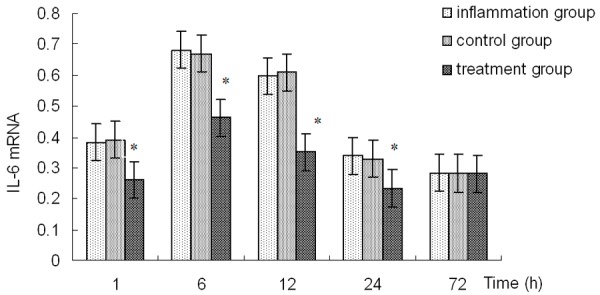
IL-6 mRNA expressions in corneas. *Indicates a significant difference from the inflammation group at P<0.01.
Effect of emodin on activation of NF-κB
The expression of p65 protein, the most studied member of NF-κB/Rel proteins, in cellular nucleus, was detected for evaluating the NF-κB activation. Western blot performed on extraction form LPS-infected corneas showed that a significant p65 protein signal was observed in corneas of the inflammation group especially at 6 h after LPS administration. No significant difference in expression of p65 protein was detected between the control group and inflammation group (P>0.05). The increased expression of p65 protein could be partly inhibited in the group pretreated with emodin (P<0.01) (Figure 6).
Figure 6.
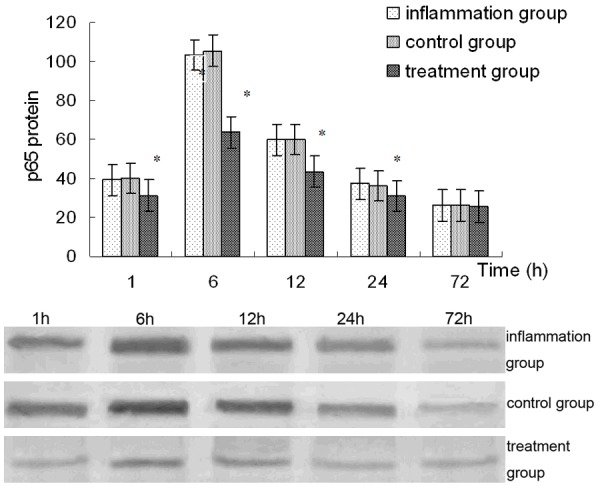
NF-κB p65 protein expressions in corneas. *Indicates a significant difference from the inflammation group at P<0.01.
Discussion
Bacterial keratitis induced by Pseudomonas aeruginosa is a rapidly progressive ocular disease, and often causes corneal perforation within several days post-infection. Animal models of bacterial keratitis continue to be of value in study of this disease and these models have led to increased understanding of the mechanisms of corneal inflammation during bacterial keratitis [11-13]. LPS, a major component of the cell wall of gram negative bacteria, is a key factor in producing numerous cytokines and causing inflammatory response in various tissues, including cornea [13-15]. It is often used to establish various animal models of inflammation [16,17]. In this study, LPS was dropped to the scarified cornea to induce acute keratitis by destroying the corneal epithelial barrier. Typical manifestations of acute keratitis, such as hyperemia, corneal edema and opacity were observed in rat’s model, and the tissue and cellular structures were found to be destroyed in rats with LPS-induced keratitis.
The cornea protects the eye from insults caused by all kinds of external factors, and an avascular and transparent cornea is required for proper vision. The integrity of the corneal epithelium is the first barrier defense to external invasion and the guarantee of corneal transparency. Cell junction plays an important role in the formation and maintenance of epithelial barrier and homeostasis in many types of epithelia including the cornea [18,19]. Tight junctions, the major apical structures in epithelium and endothelium, have been recently reported to play important roles in barrier function by forming cell-to-cell contacts and sealing paracellular pathway. Recent researches suggested that emodin could promote the expression of occludin, a major component of the tight junction, and attenuate inflammation, reduce pancreatic paracellular permeability in rats with acute pancreatitis [20,21]. The effect of emodin on corneal barrier function has not been reported. In the present study, the role of emodin as an anti-inflammatory agent in the rat cornea was investigated using a LPS-induced keratitis model. We observed that the typical manifestations of acute keratitis were improved in those rats pretreated with emodin. The decrease of infiltration was in accordance with the increased expression of occludin. Less polymorphonuclear neutrophil infiltration and lighter damages in corneas were observed in the treatment group than those in the control and inflammation group. So we think that emodin could reduce corneal inflammation and maintain the normal cellular structure and tissue condition in keratitis rats, and this anti-inflammatory effect might act through modulation of the tight junction protein, maintenance of the corneal barrier and reduction of the infiltration of inflammatory cells.
NF-kB is a ubiquitous transcription factor that, through target genes, regulates key processes such as inflammation, apoptosis, stress response, wound healing, angiogenesis, and lymphangiogenesis [22]. NF-κB/Rel proteins consist of p50, p52, c-Rel, RelB, and p65 (RelA). The p65, the most commonly studied member, interacts with NF-κB inhibitor protein (IκB) in the cytoplasm [23]. Upon activation by various types of pathological or physiological stimulation, IκB is phosphorylated and degraded. The p65 then translocates to the cellular nucleus and regulate the transcription of NF-κB target genes [4]. Thus the expression of p65 protein in cell nucleus could be used for evaluating the activation of NF-κB. The transcription factor NF-κB plays an important role in intracellular signaling induced by LPS and is therefore a potential target for new therapeutic approaches to inflammatory diseases. Its inhibitors intended to block NF-κB activity may be useful as anti-inflammatory agents [24]. One of the mechanisms of anti-inflammatory effects of emodin has been shown to be the inhibitor of NF-κB activation [25]. Here in the present study, Western blot was used to test whether emodin could inhibit the activation of NF-κB induced by LPS in rats. Results indicated that emodin could down-regulate the expression of p65 protein in corneas of rats with LPS-induced keratitis. Therefore, anti-inflammatory effects of emodin are exerted, at least in part, through the inhibition of NF-κB.
Investigations showed that many cytokines could elevate in pathological progress of keratitis corneas, and this interrelated with the degree of corneal injury directly. Gene expression of cytokines was known to be regulated by transcription factors such as NF-κB. Emodin could decrease the activation of NF-κB and the levels of cytokines [26,27]. To investigate whether the inhibitory action of emodin on the gene expression of IL-6 can be induced through inactivation of NF-κB, LPS was used as inflammation inducers in this study. Results showed that the changes of the inflammatory manifestations and infiltration degree were in accordance with the expression of IL-6 in corneal tissue of keratitis rats, which was considerably reduced in emodin pretreated corneas. Therefore, decrease in IL-6 expression with emodin was most likely, responsible for the decrease in infiltration of neutrophils and improvement of corneal damages.
In summary, we demonstrated that NF-κB pathway was up-regulated in a rat model of LPS-induced keratitis and emodin could inhibit this activation partly; pretreatment with emodin could protect against corneal inflammatory response, by reducing neutrophils infiltration and cytokines production and protecting the corneal epithelial barrier. Taken together, these data together indicated that emodin reduce corneal inflammation in LPS induced keratitis in Wistar rats may due to its capability of inhibition in NF-κB activation.
Acknowledgements
This work was supported by the Science and Technology Development Plan of Shandong Province, China (No. 2014GSF118006).
Disclosure of conflict of interest
None.
References
- 1.Oldenburg CE, Lalitha P, Srinivasan M, Manikandan P, Bharathi MJ, Rajaraman R, Ravindran M, Mascarenhas J, Nardone N, Ray KJ, Glidden DV, Acharya NR, Lietman TM. Moxifloxacin susceptibility mediates the relationship between causative organism and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2013;54:1522–1526. doi: 10.1167/iovs.12-11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somabhai Katara R, Dhanjibhai Patel N, Sinha M. A clinical microbiological study of corneal ulcer patients at western Gujarat, India. Acta Med Iran. 2013;51:399–403. [PubMed] [Google Scholar]
- 3.Huang Y, Lu Y, Zhang L, Yan J, Jiang J, Jiang H. Perineural dexmedetomidine attenuates inflammation in rat sciatic nerve via the NF-κB pathway. Int J Mol Sci. 2014;15:4049–4059. doi: 10.3390/ijms15034049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan W, Petznick A, Heryati S, Rifada M, Tong L. Nuclear Factor-κB: central regulator in ocular surface inflammation and diseases. Ocul Surf. 2012;10:137–148. doi: 10.1016/j.jtos.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 6.Alisi A, Pastore A, Ceccarelli S, Panera N, Gnani D, Bruscalupi G, Massimi M, Tozzi G, Piemonte F, Nobili V. Emodin prevents intrahepatic fat accumulation, inflammation and redox status imbalance during diet-induced hepatosteatosis in rats. Int J Mol Sci. 2012;13:2276–2289. doi: 10.3390/ijms13022276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng G, Liu Y, Lou C, Yang H. Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-κB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br J Pharmacol. 2010;161:1628–1644. doi: 10.1111/j.1476-5381.2010.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Zeng K, Qiu Y, Yan F, Lin C. Therapeutic effect of emodin on collagen-induced arthritis in mice. Inflammation. 2013;36:1253–1259. doi: 10.1007/s10753-013-9663-6. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, Pearlman E. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girgis DO, Sloop GD, Reed JM, O’Callaghan RJ. A new topical model of Staphylococcus corneal infection in the mouse. Invest Ophthalmol Vis Sci. 2003;44:1591–1597. doi: 10.1167/iovs.02-0656. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki Y, Suzuki T, Oka N, Zhang Y, Hayashi Y, Hayashi N, Gotoh N, Ohashi Y. Pseudomonas aeruginosa MucD protease mediates keratitis by inhibiting neutrophil recruitment and promoting bacterial survival. Invest Ophthalmol Vis Sci. 2014;55:240–246. doi: 10.1167/iovs.13-13151. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, McClellan SA, Barrett R, Foldenauer M, Hazlett LD. HGF Signaling Impacts Severity of Pseudomonas aeruginosa Keratitis. Invest Ophthalmol Vis Sci. 2014;55:2180–2190. doi: 10.1167/iovs.13-13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foldenauer ME, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Substance P affects growth factors in Pseudomonas aeruginosa-infected mouse cornea. Cornea. 2012;31:1176–1188. doi: 10.1097/ICO.0b013e31824d6ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta D, Du Y, Piluek J, Jakub AM, Buela KA, Abbott A, Schuman JS, SundarRaj N. Ethyl pyruvate ameliorates endotoxin-induced corneal inflammation. Invest Ophthalmol Vis Sci. 2012;53:6589–6599. doi: 10.1167/iovs.11-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chian CF, Chiang CH, Chuang CH, Liu SL. Inhibitor of nuclear factor-κB, SN50, attenuates lipopolysaccharide-induced lung injury in an isolated and perfused rat lung model. Transl Res. 2014;163:211–220. doi: 10.1016/j.trsl.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Kalariya NM, Shoeb M, Reddy AB, Sawhney R, Ramana KV. Piceatannol suppresses endotoxin-induced ocular inflammation in rats. Int Immunopharmacol. 2013;17:439–446. doi: 10.1016/j.intimp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Chang CY, Fu E, Chiang CY, Chang WJ, Cheng WC, Tu HP. Effect of paeonol on tissue destruction in experimental periodontitis of rats. Am J Chin Med. 2014;42:361–374. doi: 10.1142/S0192415X14500244. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz-Melo MT, Sánchez-Guzmán E, González-Robles A, Valdés J, Gómez-Flores E, Castro-Muñozledo F. Expression of claudins -2 and -4 and cingulin is coordinated with the start of stratification and differentiation in corneal epithelial cells: retinoic acid reversibly disrupts epithelial barrier. Biol Open. 2013;15:132–143. doi: 10.1242/bio.20123145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J, Huang J, Chen C, Gao N, Wang F, Yu FS. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:6589–6596. doi: 10.1167/iovs.11-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia XM, Wang FY, Wang ZK, Wan HJ, Xu WA, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol. 2010;16:2994–3001. doi: 10.3748/wjg.v16.i24.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia XM, Li BK, Xing SM, Ruan HL. Emodin promoted pancreatic claudin-5 and occludin expression in experimental acute pancreatitis rats. World J Gastroenterol. 2012;18:2132–2139. doi: 10.3748/wjg.v18.i17.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartuzi P, Hofker MH, van de Sluis B. Tuning NF-κB activity: a touch of COMMD proteins. Biochim Biophys Acta. 2013;1832:2315–2321. doi: 10.1016/j.bbadis.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Jeong YT, Li X, Kim MJ, Park PH, Hwang SL, Son JK, Chang HW. Emodin Isolated from Polygoni cuspidati Radix Inhibits TNF-α and IL-6 Release by Blockading NF-κB and MAP Kinase Pathways in Mast Cells Stimulated with PMA Plus A23187. Biomol Ther (Seoul) 2013;21:435–441. doi: 10.4062/biomolther.2013.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Shitany NA, El-Bastawissy EA, El-Desoky K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 Via an antioxidant mechanism. Int Immunopharmacol. 2014;19:290–299. doi: 10.1016/j.intimp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Kitano A, Saika S, Yamanaka O, Ikeda K, Okada Y, Shirai K, Reinach PS. Emodin suppression of ocular surface inflammatory reaction. Invest Ophthalmol Vis Sci. 2007;48:5013–5022. doi: 10.1167/iovs.07-0393. [DOI] [PubMed] [Google Scholar]
- 26.Song ZC, Wang ZS, Bai JH, Li Z, Hu J. Emodin, a naturally occurring anthraquinone, ameliorates experimental autoimmune myocarditis in rats. Tohoku J Exp Med. 2012;227:225–230. doi: 10.1620/tjem.227.225. [DOI] [PubMed] [Google Scholar]
- 27.Li A, Dong L, Duan ML, Sun K, Liu YY, Wang MX, Deng JN, Fan JY, Wang BE, Han JY. Emodin improves lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Microcirculation. 2013;20:617–628. doi: 10.1111/micc.12061. [DOI] [PubMed] [Google Scholar]


