Abstract
Objective: This study aims to explore the effects of the traditional Chinese medicine monomer cryptotanshinone (CTS) on the expression levels of inflammatory factors in myocardial cells caused by Ang II and its mechanism. Methods: The neonatal rat myocardial cells were cultured in vitro in this study. Their purities were identified by immunocytochemical method. The cellular viability in different groups was determined by MTT assay. The levels of TNF-α and IL-6 in the supernatant of cell culture were detected with ELISA method. The levels of intracellular reactive oxygen species (ROS) were detected by Dihydrogen ethidium (DHE) staining method. The location changes of NF-κB in cells were detected by immunofluorescence method. Results: The purity of primary cultured neonatal rat myocardial cells was over 95%, CTS had no obvious effect on the viability of cells while it inhibited the increased levels of TNF-α, IL-6 and ROS caused by Ang II with dose dependent. NF-κB mainly distributed in the cytoplasmic region in normal cells, it translocated to the nucleus after Ang II stimulation while CTS inhibited the translocation. Conclusions: CTS could inhibit the inflammatory factors such as TNF-α and IL-6 in myocardial cells induced by Ang II with dose dependent, its mechanism may be related with that CTS could decrease the levels of ROS in myocardial cells and inhibit NF-κB translocation into the nucleus.
Keywords: Cryptotanshinone (CTS), Ang II, TNF-α, IL-6, NF-κB
Introduction
Inflammation plays an important role in the occurrence and development of cardiovascular disease (CVD). Inflammatory cytokines have dual effects, on the one hand they play an important protecting role in the immune regulation and anti infection, on the other hand, they may cause inflammatory reaction cascade which is harm to the cells if sustained release or produce too much. The sustained release of tumor necrosis factor-alpha (TNF-α) could directly damage myocardial cells and increase the susceptibility of patients to myocarditis [1]. Kanda reported that interleukin-6 (IL-6) in the blood had close correlation with the left ventricular dysfunction [2]. Oxidative stress is a feature of inflammatory reaction [3-7] and reactive oxygen species (ROS) include the small molecules closely related to oxidative stress. The nuclear transcription factors sensitive to activated oxygen in cells include nuclear factor kappa B (NF-κB) and activated protein-1 (AP-1) etc. Studies showed that NF-κB was strongly activated in the process of heart failure [8], myocardial ischemic reperfusion, unstable angina, atherosclerosis and others, it plays the role in CVD by regulating the expression of adhesion molecules and inflammatory molecules, etc [9].
Angiotensin II (Ang II) binding with its receptor can increase intracellular ROS [10] and activate multiple transcription factors, such as NF-κB, AP-1, signal transcription and activation factor (STAT) and cAMP reactive binding protein (CREB) [11,12], which mediated the production of TNF-α, IL-6, IFN-γ and other inflammatory mediators [13-15]. Study on the relationship between Ang II and ROS and NF-κB may provide a new theoretical basis for the pathogenesis and treatment of CVD.
Salvia miltiorrhiza is a kind of traditional Chinese medicine and was widely used in the treatment of coronary heart disease and other cardiovascular and cerebrovascular diseases. Tanshinone is the effective active component of salvia miltiorrhiza including tanshinone I, tanshinone IIA, tanshinone IIB, cryptotanshinone (CTS) and other more than 10 monomer. Studies showed that CTS can reduce the elevated expression of MMP-9 induced by TNF-α and the increased activity of COX-2 induced by LPS [16,17]. Our previous study found that CTS could inhibit the inflammatory response of macrophages [18]. There were no reports on the intervention effect of CTS on the inflammatory factors. Therefore, we studied the intervention effects of CTS on myocardial inflammatory reaction induced by Ang II and its possible mechanism in this study.
Materials and methods
Culture and identification of neonatal rat primary myocardial cells
Heart was taken out from 2~3 days SD rat and put into dish with cooling PBS. The heart was cut into 1 mm3 pieces after removing the connective tissue on the heart. The pieces were washed and put into a flask containing a rotor. They were digested with 10~15 ml 0.1% trypsin and 200 ul DNA enzyme (0.05 mg/ml) on ice for 20 min. Then the flask was put on a magnetic stirrer at 37°C for 2 h. They were centrifuged at 1000 rpm for 5 min and washed with PBS. The isolated cells were cultured in DMEM medium containing 10% fetal bovine serum at 37°C with 5% CO2. The culture medium was collected after 1 hours and 0.1 mmol/l BrdU (10 mL/mL) was added to inhibit fibroblast proliferation. Then they were cultured in plates for 3-9 days. The morphology, adherent and pulse rhythm of myocardial cells were observed under inverted phase contrast microscope. The purity of cultured myocardial cells was identified using the anti-α-sarcomeric actin antibody. They were performed using immunohistochemistry staining kit according to the manual.
Detection of the vitality of myocardial cells by MTT assay
Myocardial cells (2 × 105/ml) were inoculated in 96-well plate and cultured for 72 h. Then they were treated with 0.1% DMSO, 1 μM Ang II and 10 mM CTS respectively and cultured for 24 h. 20 μL MTT (5 mg/ml) was added into each well and cultured at 37°C for 4 h. The medium was discarded and 150 μL DMSO was added into them. OD values at 570 nm were determined.
Detection of TNF-α and IL-6 levels with enzyme linked immunosorbent assay (ELISA)
The myocardial cells were treated with CTS (2.5 μM, 5 μM, 10 μM) for 1 h respectively after culture for 72 h. Then they were treated with 100 nM Ang II and cultured for 24 h. The culture supernatant was collected and TNF-α and IL-6 levels were detected with ELISA kit according to the manual. OD values at 450 nm were determined by ELISA detector. Standard curve was drawn to calculate the concentration of TNF-α and IL-6 in the samples.
Detection of intracellular ROS
The myocardial cells were treated with CTS (2.5 μM, 5 μM, 10 μM) for 1 h respectively after culture for 72 h. Then they were treated with 100 nM Ang II and cultured for 24 h. 10 μmol/L dihydrogen ethidium (DHE) was added into them and incubated in dark at 37°C for 30 min. The cells were washed two times with PBS and ROS were observed under fluorescence microscope.
Detection of NF-κB location with immunofluorescence method
Myocardial cells (2 × 105/ml) were inoculated in 6-well plate and cultured for 72 h. They were treated with CTS (2.5 μM, 5 μM, 10 μM) for 1 h respectively, Ang II was added into them after that and incubated for 30 min. The medium was discarded and the cells were washed with PBS. The cells were fixed with 4% paraformaldehyde for 30 min at room temperature. Discard the paraformaldehyde and air dry for 10 min. They were treated with PBS containing 0.5% Triton X-100 for 15 min and normal nonimmune animal serum was added into them and incubated for 10 min at room temperature. The serum was discarded and washed with PBS, 200 μL (1:100 dilution) mouse anti NF-κB (p65) was added into them and incubated at 4°C overnight. They were washed with PBS and fluorescein labeled second antibody (100 μL, 1:200) was added into them and incubated at 37°C for 30 min. The nucleus was stained with Hochest 33342 (1:500). NF-κB was stained green fluorescence and nucleus was blue.
Statistical analysis
All statistical analyses were performed with SPSS 13.0 statistical software. Data were expressed as means ± standard deviations (SD). Differences among groups were analyzed using t test and one-way ANOVA. P < 0.05 were considered statistically significant.
Results
The identification of myocardial cells and determination of vitality
The cultured adherent myocardial cells were spindle, triangular or irregular polygon, the nucleus was elliptic, the pseudopodia among cells contact with each other and interwoven into a network and gradually formed a cell cluster or cell monolayer (Figure 1A). The primary myocardial cells began to beat rhythmically after culture for three days, there was no difference in the beat frequency between the third day and the ninth day (Table 1, P > 0.05). The immunohistochemical staining of α-sarcomeric actin showed that it was positive (Figure 1B). There was no brown positive cell in control group (Figure 1C, 1D).
Figure 1.
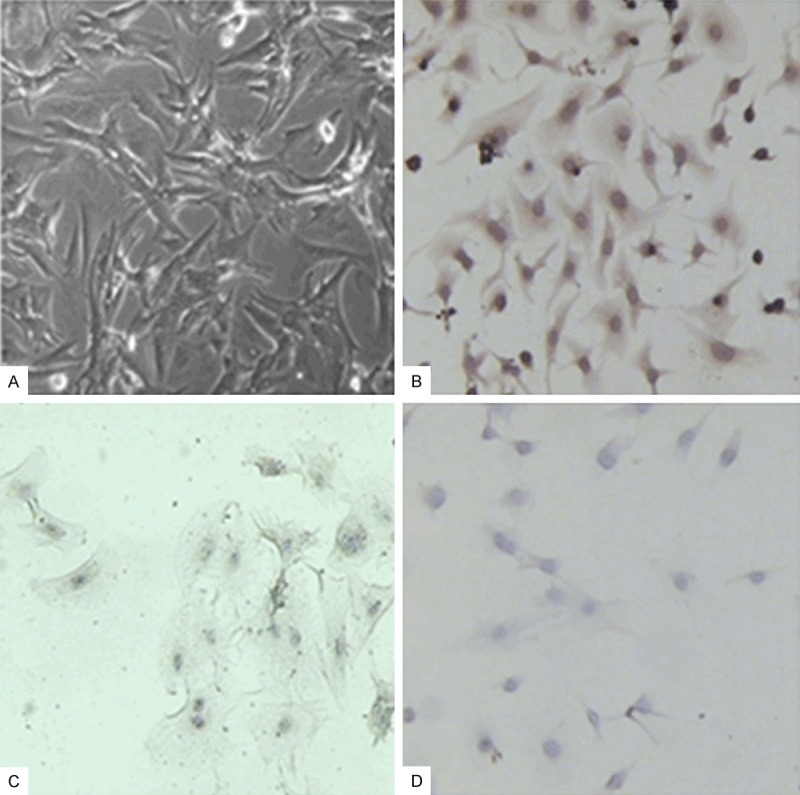
The culture and identification of myocardial cells. A. Myocardial cells cultured for 72 h (×200); B. The immunohistochemical staining of α-sarcomeric actin in myocardial cells (72 h, ×200); C. The immunohistochemical staining of α-sarcomeric actin in myocardial fibroblasts (72 h, ×200); D. The negative control (72 h, ×200).
Table 1.
Vitality test of cultured Myocardial cells (x̅ ± s, n = 20)
| Time (day) | Pulse (times/min) |
|---|---|
| 3 | 85.20 ± 2.27 |
| 5 | 85.30 ± 2.61 |
| 7 | 84.20 ± 2.41 |
| 9 | 84.30 ± 1.75 |
The effects on the vitality of myocardial cells
The effects on the activity of myocardial cells were detected by MTT assay. We found that 0.1% DMSO had no obvious effect on the activity of myocardial cells, 1 μM Ang II and 10 μM CTS also had no obvious effect on the activity of myocardial cells (Figure 2; Table 2, P > 0.05). So the drug concentration in this experiment was safe without cytotoxicity.
Figure 2.
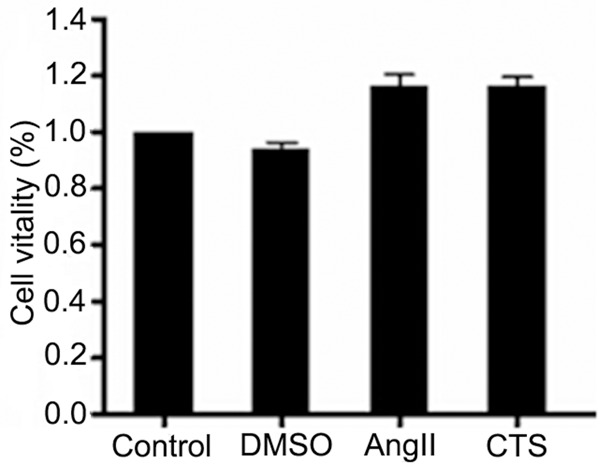
The effects of drugs on the activity of myocardial cells (x̅ ± s, n = 6).
Table 2.
The effects on activity of myocardial cells (x̅ ± s, n = 6)
| Group | OD values |
|---|---|
| Control | 0.715 ± 0.065 |
| 0.1% DMSO | 0.679 ± 0.047 |
| 1 μM Ang II | 0.822 ± 0.051 |
| 10 μM CTS | 0.815 ± 0.067 |
Detection of the TNF-α and IL-6 levels in the cell culture supernatant
The results of ELISA showed that the TNF-α and IL-6 levels in control group was low while they increased significantly in Ang II group (P < 0.05), the TNF-α and IL-6 levels in 2.5 μM, 5 μM and 10 μM CTS group were significantly lower than that of Ang II group (P < 0.05), it suggested that CTS could inhibit the increased TNF-α and IL-6 levels induced by Ang II with dose dependent (Figure 3; Table 3).
Figure 3.
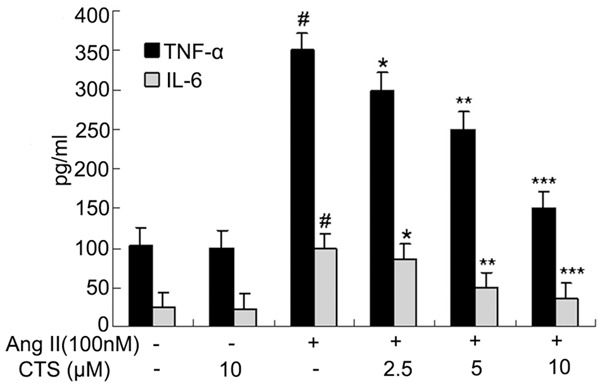
The effects on the TNF-α and IL-6 levels in the cell culture supernatant. Compared with control group, #P < 0.05; Compared with Ang II group, *P < 0.05, **P < 0.01, ***P < 0.001.
Table 3.
The levels of TNF-α and IL-6 in the cell culture supernatant (x̅ ± s, n = 6, pg/mL)
| Group | TNF-α | IL-6 |
|---|---|---|
| Control | 100 ± 3.72 | 30 ± 1.52 |
| 10 μM CTS | 99 ± 3.49 | 29 ± 1.43 |
| Ang II | 345 ± 10.82 | 100 ± 4.96 |
| Ang II + 2.5 μM CTS | 305 ± 8.74 | 88 ± 3.49 |
| Ang II + 5 μM CTS | 251 ± 7.84 | 50 ± 2.47 |
| Ang II + 10 μM CTS | 156 ± 4.67 | 39 ± 2.38 |
Detection of the ROS levels
A small amount of red fluorescence can be detected in normal cell, it suggested that the intracellular ROS level is relatively low. The fluorescence in the cell increased significantly after the cell was treated with 100 nM Ang II for 24 h, which suggested that Ang II could increase the intracellular ROS level. The fluorescence in the cell in 10 μM CTS group was similar with that of control group, CTS could inhibit the increased ROS level induced by Ang II with dose dependent (Figure 4).
Figure 4.
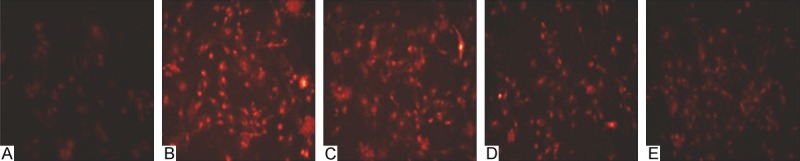
The effects on the intracellular ROS levels. A. Control group (×200); B. 100 nM Ang II group (×200); C. 2.5 μM CTS + 100 nM Ang II group (×200); D. 5 μM CTS + 100 nM Ang II group (×200); E. 10 μM CTS + 100 nM Ang II group (×200).
The translocation of NF-κB
Immunofluorescence showed that NF-κB mainly existed in the cytoplasm in normal myocardial cells with little in nuclear area, which suggested that NF-κB (p65) was mainly in a static state. NF-κB obviously aggregated in the nucleus and near the nuclear membrane area after the cells were treated by Ang II. There was NF-κB both in cytoplasmic and nuclear area in 2.5 μM CTS group, while in 5 μM and 10 μM CTS group, NF-κB mainly existed in cytoplasm. So CTS inhibited the translocation of NF-κB (Figure 5; Table 4).
Figure 5.
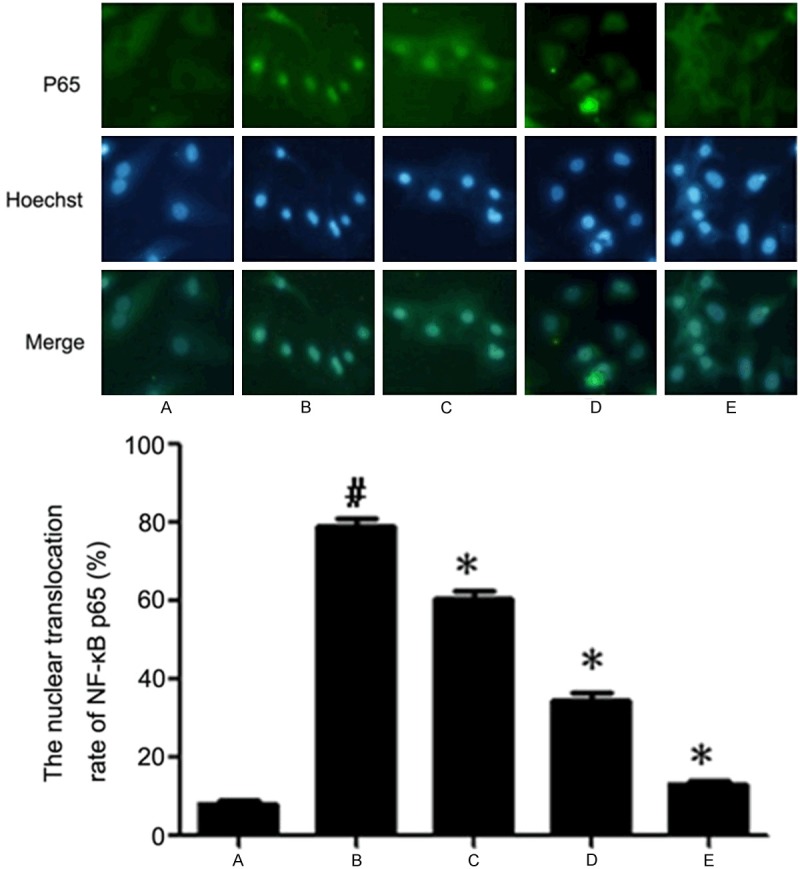
The nuclear translocation rate of NF-κB (p65) in myocardial cells. A. Control group; B. 100 nM Ang II group; C. 2.5 μM CTS + 100 nM Ang II group; D. 5 μM CTS + 100 nM Ang II group; E. 10 μM CTS + 100 nM Ang II group. Compared with control group, #P < 0.05; Compared with Ang II group, *P < 0.05.
Table 4.
The nuclear translocation rate of NF-κB (p65) in myocardial cells
| Group | Nuclear translocation rate (%) |
|---|---|
| Control | 8 ± 1.53 |
| Ang II | 80 ± 3.93 |
| Ang II + 2.5 μM CTS | 64 ± 2.15 |
| Ang II + 5 μM CTS | 38 ± 3.51 |
| Ang II + 10 μM CTS | 15 ± 1.15 |
Discussion
The myocardial cells cultured in vitro can maintain certain characteristics of the structure and function of in vivo myocardial. They had the advantages of simple, quantitative, reproducible and not affected by neurohumoral factors. In this study, we used the improved myocardial cell culture method and cells had high yield and good active state. The use of in vitro cell model can confirm the direct effect of Ang II on myocardial cells.
Ang II as a factor with the roles of vascular contraction and promoting proliferation can increase blood pressure and activate mononuclear phagocyte system to release a variety of inflammatory factor, which could cause leukocyte adhesion, induce the apoptosis of endothelial cell and atherosclerosis and promote myocardial remodeling. In this study, we found that Ang II could increase the levels of TNF-α and IL-6 in the myocardial cell culture supernatant while CTS could inhibit this effect of Ang II with dose dependent. Ang II could increase the levels of ROS in cells. Our previous study showed that CTS had obvious protective effects on glutamate toxicity of cultured nerve cells, its mechanism was related with CTS against the role of oxidative stress induced by glutamate [18]. In this study, we observed that CTS could reduce the increased ROS levels induced by Ang II with dose dependent. NF-κB binding with inhibition protein monomer IκB at resting state existed in cytoplasm as inactive forms. The protein kinase in cytosol can be activated by intracellular ROS and caused the dissociation of NF-κB and IκB, NF-κB transferred into the nucleus and combined specifically with the κB sequence in immunoglobulin κ light chain, increased the expression of inflammatory factors such as TNF-α and IL-6 gene [13-15]. In this study, it is obvious of NF-κB into the nucleus after the myocardial cells were treated by Ang II for 30 min, while CTS could inhibit this effect of Ang II with dose dependent.
In a word, we concluded that CTS could reduce the TNF-α and IL-6 levels in the cell culture supernatant, this may be related with that CTS could decrease the levels of ROS in myocardial cells and inhibit NF-κB translocation into the nucleus.
Acknowledgements
This study was supported by the science and technology research projects of Education Department of Henan province (12A350006).
Disclosure of conflict of interest
None.
References
- 1.Kim HJ, Tsoy I, Park JM, Chung JI, Shin SC, Chang KC. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006;580:1391–1397. doi: 10.1016/j.febslet.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 2.Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45:183–193. doi: 10.1536/jhj.45.183. [DOI] [PubMed] [Google Scholar]
- 3.Yu X, Tao W, Jiang F, Li C, Lin J, Liu C. Celastrol attenuates hypertension-induced inflammation and oxidative stress in vascular smooth muscle cells via induction of heme oxygenase-1. Am J Hypertens. 2010;23:895–903. doi: 10.1038/ajh.2010.75. [DOI] [PubMed] [Google Scholar]
- 4.Shannahan JH, Schladweiler MC, Richards JH, Ledbetter AD, Ghio AJ, Kodavanti UP. Pulmonary oxidative stress, inflammation, and dysregulated iron homeostasis in rat models of cardiovascular disease. J Toxicol Environ Health A. 2010;73:641–656. doi: 10.1080/15287390903578208. [DOI] [PubMed] [Google Scholar]
- 5.Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, Singal PK. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. 2010;13:1033–1049. doi: 10.1089/ars.2009.2930. [DOI] [PubMed] [Google Scholar]
- 6.McCall DO, McGartland CP, McKinley MC, Sharpe P, McCance DR, Young IS, Woodside JV. The effect of increased dietary fruit and vegetable consumption on endothelial activation, inflammation and oxidative stress in hypertensive volunteers. Nutr Metab Cardiovasc Dis. 2011;21:658–664. doi: 10.1016/j.numecd.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Pallares J, Rey P, Parga JA, Munoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol. 2008;375:637–649. doi: 10.1016/j.jmb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton JW, White DJ, Maass DL, Hybki DP, Haudek S, Giroir B. Antioxidant vitamin therapy alters burn trauma-mediated cardiac NF-kappaB activation and cardiomyocyte cytokine secretion. J Trauma. 2001;50:397–406. doi: 10.1097/00005373-200103000-00002. discussion 407-398. [DOI] [PubMed] [Google Scholar]
- 10.Tu VC, Sun H, Bowden GT, Chen QM. Involvement of oxidants and AP-1 in angiotensin II-activated NFAT3 transcription factor. Am J Physiol Cell Physiol. 2007;292:C1248–1255. doi: 10.1152/ajpcell.00624.2005. [DOI] [PubMed] [Google Scholar]
- 11.Guo RW, Yang LX, Wang H, Liu B, Wang L. Angiotensin II induces matrix metalloproteinase-9 expression via a nuclear factor-kappaB-dependent pathway in vascular smooth muscle cells. Regul Pept. 2008;147:37–44. doi: 10.1016/j.regpep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Holownia A, Braszko JJ. The effect of angiotensin II and IV on ERK1/2 and CREB signalling in cultured rat astroglial cells. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:157–163. doi: 10.1007/s00210-007-0192-4. [DOI] [PubMed] [Google Scholar]
- 13.Guo F, Chen XL, Wang F, Liang X, Sun YX, Wang YJ. Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. J Interferon Cytokine Res. 2011;31:351–361. doi: 10.1089/jir.2010.0073. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294:E345–351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 15.Fang C, Liu Y, Lu P, Liang Q, Liu X, Zhao W, Su W. Total saponins of panax ginseng inhibiting human endothelium cells’ damages induced by angiotensin II via AT1 receptor. J Ethnopharmacol. 2011;138:439–444. doi: 10.1016/j.jep.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Jin DZ, Yin LL, Ji XQ, Zhu XZ. Cryptotanshinone inhibits cyclooxygenase-2 enzyme activity but not its expression. Eur J Pharmacol. 2006;549:166–172. doi: 10.1016/j.ejphar.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK, Lee SH, Jeon SJ, Son KH, Chang YC, Lee YC, Kim CH. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-alpha-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-kappaB and AP-1. Biochem Pharmacol. 2006;72:1680–1689. doi: 10.1016/j.bcp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Tang S, Shen XY, Huang HQ, Xu SW, Yu Y, Zhou CH, Chen SR, Le K, Wang YH, Liu PQ. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW264.7 macrophages through inhibition of the NF-kappaB and MAPK signaling pathways. Inflammation. 2011;34:111–118. doi: 10.1007/s10753-010-9214-3. [DOI] [PubMed] [Google Scholar]


