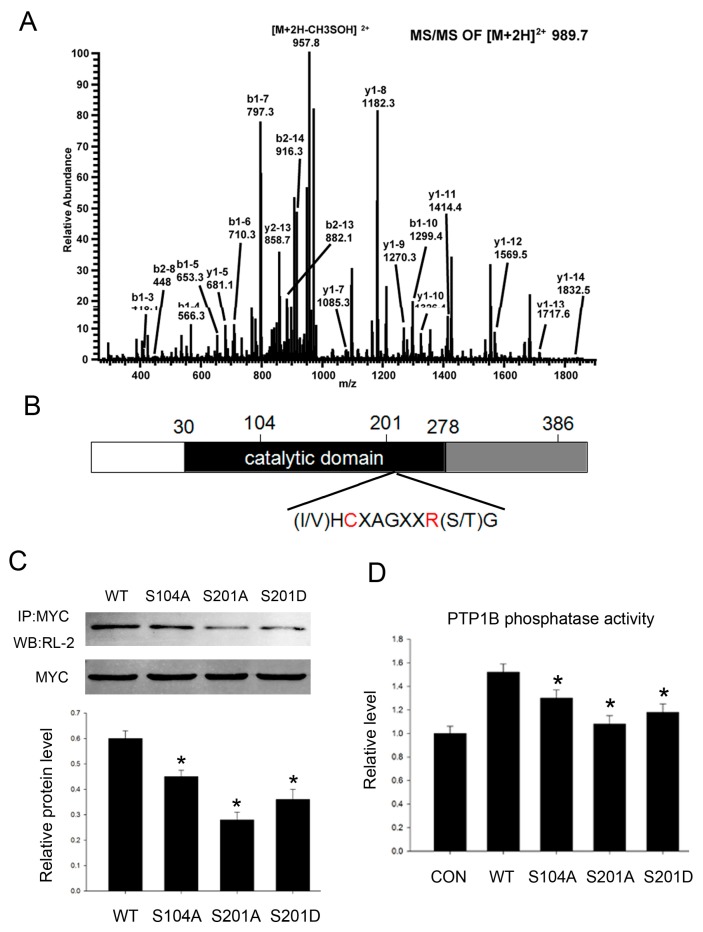
Figure 3.
PTP1B is O-GlcNAc modified at three sites. (A) ETD MS/MS site mapping of the human PTP1B O-GlcNAc modification sites; (B) Schematic diagram shows full-length PTP1B domain structure and O-GlcNAc sites (Ser104, Ser201, and Ser386); C (Cys215) and A (Arg221), two residues which are critical for the catalytic activity of PTP1B; (C) We constructed site-directed mutants (Ser104 mutated to alanine, Ser201 mutated to alanine and aspartic acid), then HepG2 cells were transfected with pcDNA3.1/myc-His (−) vectors containing the indicated site mutations for 24 h before treating with 0.2 mM palmitate acid for 18 h. Then cells were immunoprecipitated with an anti-myc antibody followed by immunoblotting with an anti-O-GlcNAc antibody (RL-2); (D) PTP1B phosphatase activity was analyzed by protein phosphatase assay kit according to the manufacturer’s instruction. Data show mean ± SEM of three independent experiments. (n = 3, * p < 0.05, significantly different from respective controls).