Abstract
Citrobacter rodentium is a murine pathogen that serves as a model for enteropathogenic Escherichia coli. C. rodentium infection reduced the quantity and activity of mitochondrial respiratory complexes I and IV, as well as phosphorylation capacity, mitochondrial transmembrane potential and ATP generation at day 10, 14 and 19 post infection. Cytokine mRNA quantification showed increased levels of IFNγ, TNFα, IL-4, IL-6, and IL-12 during infection. The effects of adding these cytokines, C. rodentium and E. coli were hence elucidated using an in vitro colonic mucosa. Both infection and TNFα, individually and combined with IFNγ, decreased complex I and IV enzyme levels and mitochondrial function. However, IL-4 reversed these effects, and IL-6 protected against loss of complex IV. Both in vivo and in vitro, the dysfunction appeared caused by nitric oxide-generation, and was alleviated by an antioxidant targeting mitochondria. IFNγ −/− mice, containing a similar pathogen burden but higher IL-4 and IL-6, displayed no loss of any of the four complexes. Thus, the cytokine environment appears to be a more important determinant of mitochondrial function than direct actions of the pathogen. As IFNγ and TNFα levels increase during clearance of infection, the concomitant increase in IL-4 and IL-6 protects mitochondrial function.
Infection with the attaching and effacing (A/E) murine pathogen Citrobacter rodentium is used as a model for studying the effects of other A/E pathogens that cause human diseases, such as enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC)1,2,3. C. rodentium infection causes colitis characterised by crypt hyperplasia, goblet cell depletion and the presence of transmural inflammatory infiltrate4. In concert with these features, enhanced crypt epithelial cell death is also observed both in C. rodentium infected colon and in E coli infected human epithelial cells1,5,6,7.
Mitochondria play pivotal roles in cell function, providing most of the cell’s energy and participating in the Ca2+, redox and pH homeostasis8,9. Thus, major mitochondrial dysfunction is likely to make the cells more susceptible to factors leading to cell death. Several inter-related mitochondrial pathways regulate cell death processes, mainly by disrupting the mitochondrial respiratory chain resulting in a decrease in adenosine triphosphate (ATP) production; opening the mitochondrial permeability transition pore causing dissipation of membrane potential; release of cytochrome c; alteration of the cell’s redox status; and overproduction of reactive oxygen species8,9.
C. rodentium uses the same machinery as A/E E. coli to infect the host, attaching to the surface of intestinal epithelial cells through formation of a type III secretion system (T3SS)1,2,3. These bacteria use the T3SS to inject effector proteins, including the mitochondrial associated protein (Map) and several virulence factors like EspF, EspG and EspH into host cells3,10,11,12. EspF and Map are known to translocate into the host mitochondria and are involved in the disruption of normal cellular physiological functions11,13,14,15. Previous studies have shown that in murine C. rodentium infection, EspF targets mitochondria to initiate the host cell death pathway by alteration of membrane potential and release of cytochrome c into the cytoplasm11,14,16. Six days after C. rodentium infection, Map was found co-localised with host mitochondria, concurrent with a decrease in immunohistological staining for succinate dehydrogenase (SDH, complex II)13. However, the effects of C. rodentium on the other mitochondrial respiratory complexes involved in the electron transport chain, complexes I, III and IV, have not been examined.
Direct attachment of bacteria or injection of bacterial effector proteins can thus cause mitochondrial dysfunction of luminal epithelial cells13,14,16 , but mitochondrial pathway mediated cell death has also been observed in basal crypt epithelial cells, even though C. rodentium are rarely found at the bottom of the crypts5. This observation raises the possibility that cytokines upregulated during infection play a role in these responses, since cytokines influence mitochondria in other pathological conditions17.
The aim of the present study was to examine the status of mitochondrial enzymes and function during infection and clearance in the murine C. rodentium infection model, and delineate the role of the bacteria per se versus cytokines induced during different time points of the infection, using an in vivo like polarised in vitro epithelial mucosal surface that secretes a mucus layer18. We found mitochondrial dysfunction in the murine colonic epithelial cells following C. rodentium infection, in particular inhibition of complex I and IV of the mitochondrial respiratory chain, and loss of mitochondrial membrane phosphorylation capacity, membrane potential and ATP generation. The in vitro experiments indicated that the mechanism behind the mitochondrial dysfunction involved interferon gamma (IFNγ), tumour necrosis factor alpha (TNFα) and C. rodentium decreasing complex I and IV quantity and activity through activation of the nitric oxide (NO) pathway. IL-4, overexpressed only during the infection clearance phase, partially abrogates the mitochondrial dysfunction by reducing enhanced NO production, signifying the beneficial role IL-4 might play during infection clearance.
Results
Infection with C. rodentium induced colitis and cell death
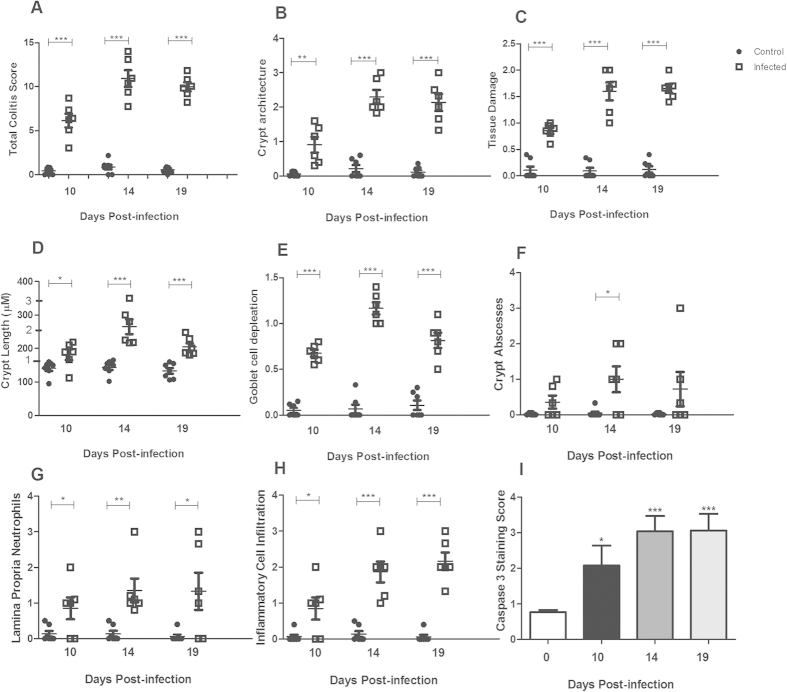
We have previously shown that in C. rodentium infected C57BL/6 mice, the highest pathogen density in the feces is reached around day 10, then starts to decrease at day 14 and finally the infection is cleared (i.e. less than 100 CFU C. rodentium/g feces) around day 1919. We therefore focused on these three time points. Infection with C. rodentium produced features typical of colitis in wild type C57BL/6 (WT) mice (P < 0.001, Fig. 1A). On day 10, infected mice had mild overall colitis (Fig. 1A), but marked goblet cell depletion (Fig. 1E). On day 14 and 19 post-infection, there was an increase in crypt length, presence of neutrophils in the lamina propria and goblet cell depletion (Fig. 1B–H). In line with previous studies demonstrating cell death and sloughing of cells during C. rodentium infection19, the presence of the active cleaved form of caspase-3, indicative of apoptosis, increased in both the luminal surface and in the crypts (Fig. 1I). The caspase-9-caspase-3 cascade is activated by pro-apoptotic molecules such as cytochrome c released from mitochondria20,21.
Figure 1. Colitis and caspase-3 staining during C. rodentium infection in the distal colon of WT mice.
(A) The total colitis score represents the sum of the individual scores of the following parameters: (B) crypt architecture, (C) tissue damage, (D) crypt length (the numbers 1–3 on the y-axes indicates how the crypt lengths were translated to scores for the incorporation into the total colitis score), (E) goblet cell depletion, (F) crypt abscesses, (G) neutrophils in lamina propria and (H) inflammatory cell infiltration. Values are mean ± S.E.M (n = 6–7 mice). Statistics: unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001 compared to uninfected control. (I) Caspase-3 quantification. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001. vs. control. The infection experiments were performed twice, and each time point contains results pooled from 4–9 mice.
Loss of immunohistochemical (IHC) staining intensity for mitochondrial respiratory enzyme complex I, II and IV in infected WT mice
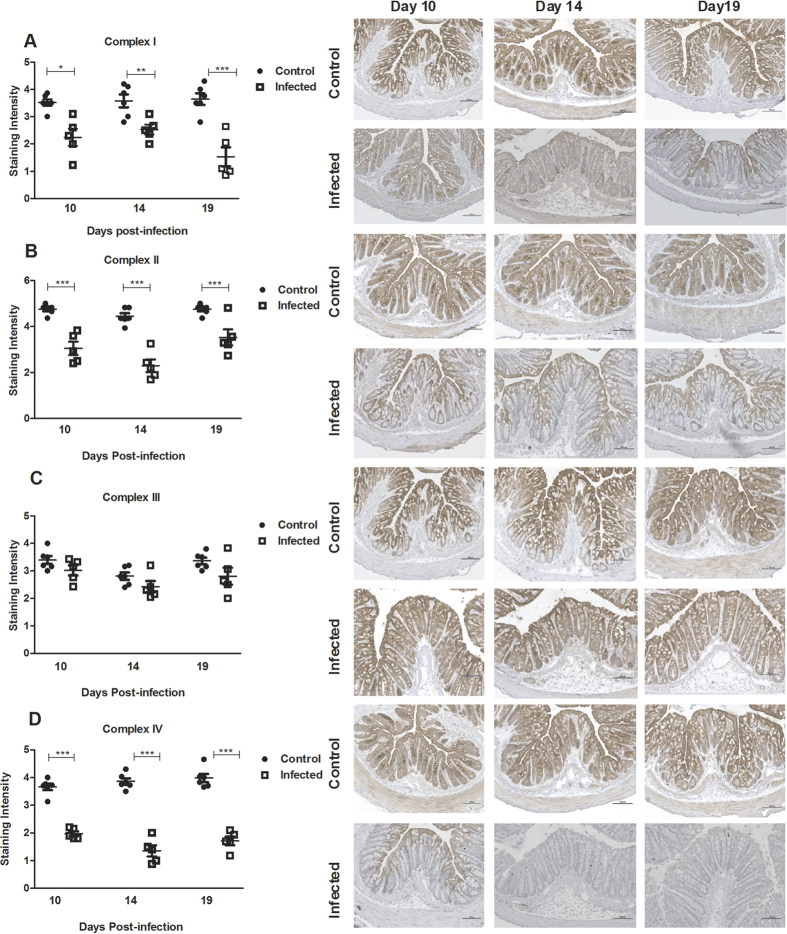
Electron microscopy has previously shown that the mitochondria are located uniformly in non-goblet cells of the colon22. In the full goblet cells, the mucin granulae displaces most of the mitochondria to the rim of the cells, and evacuation of the mucin droplets discloses a rich content of mitochondria spread throughout the cytoplasm22. In line with this, the IHC staining patterns of all four complexes (complex I-IV) were relatively uniform in the majority of the epithelial surface cells, whereas the full goblet cells displayed pale areas where the mucin granulae are present (Fig. 2). During infection (day 10, 14 and 19) with C. rodentium, the intensity of the immunohistochemical staining for complex I, II and IV decreased in the epithelial cells (P < 0.05, Fig. 2A,B and D). However, no loss of staining intensity for complex-III was observed (Fig. 2C).
Figure 2. Tissue localization and semi-quantification of the mitochondrial respiratory enzyme complexes in the murine distal colon during C. rodentium infection.
Immunohistochemical staining using antibodies for (A) MTND6 (complex I) (B) SDHA (complex-II) (C) CYC1 (complex-III) (D) CCO-VIc (complex-IV). Statistics: unpaired t test. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control, n = 5–6 mice/group. The infection experiments were performed twice, and each time point contain results pooled from 5–6 mice. Scale bar 100 μm, magnification × 200.
Infection with C. rodentium caused dysfunction of mitochondrial respiratory enzyme complexes I and IV in infected WT mice
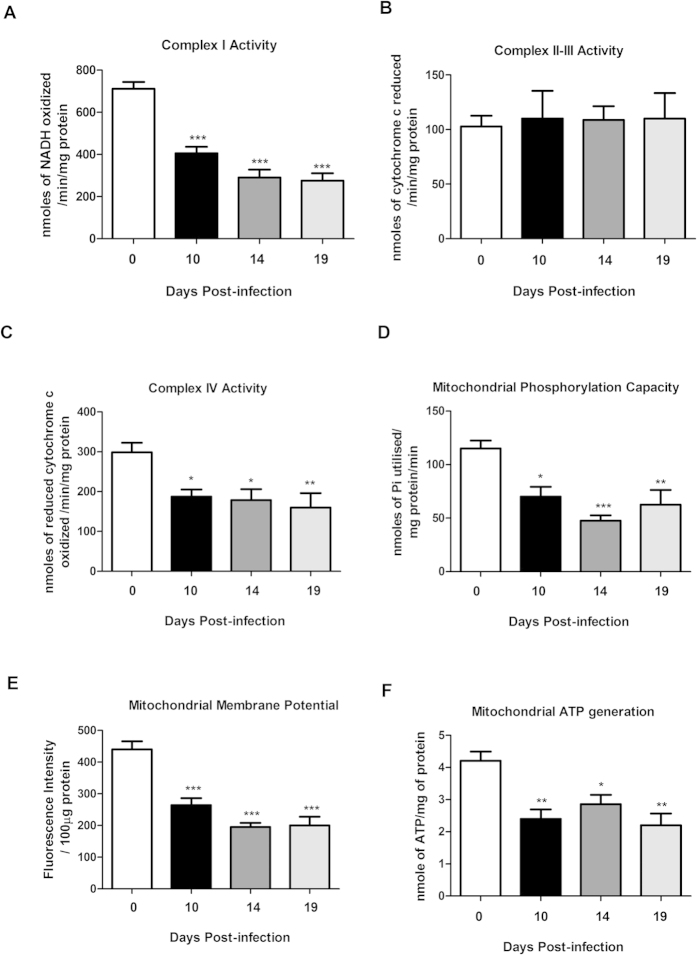
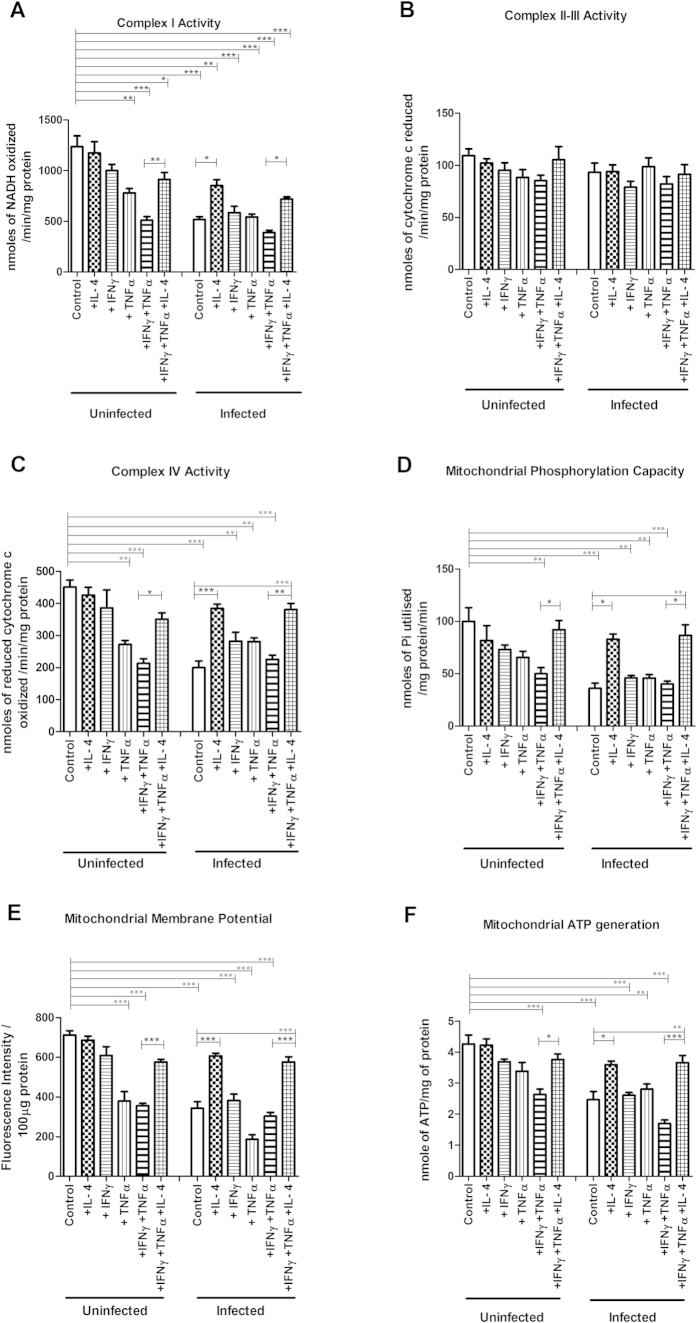
Next, we investigated if the decrease of staining intensity of mitochondrial complexes reflected their activity. Complex-I activity decreased by 43% during the mid-infection time point at 10 days post-infection, further decreased by day 14 (−59%) and remained low through to day 19 (−61%, P < 0.001, Fig. 3A). Similarly, complex-IV activity was reduced during infection (day 10: −37%, day 14: −40%, day 19: −46%, P < 0.05, Fig. 3C). No loss of enzymatic activity was observed at any time points for complex-II-III activity (Fig. 3B). In addition to the unchanged complex III protein levels and activity, we did not detect any loss in citrate synthase activity with infection (p = 0.6; control mice; 1.146 ± 0.10 U/mg protein, infected mice day 10; 1.046 ± 0.12 U/mg protein, infected mice day 14; 1.02 ± 0.026 U/mg protein). Together, this indicates that the amount of mitochondria do not decrease23, but that a loss of mitochondrial functionality occurs.
Figure 3. Mitochondrial function in the murine distal colon after C. rodentium infection.
(A) complex I activity (B) complex II-III activity (C) complex IV activity (D) mitochondrial phosphorylation capacity (E) mitochondrial Membrane potential (F) mitochondrial ATP generation. Values are mean ± S.E.M. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. (n = 4–9 mice/group). The infection experiments were performed twice, and each time point contains results pooled from 4–9 mice.
C. rodentium infection caused a reduction of phosphorylation capacity, mitochondrial transmembrane potential and ATP generation in infected WT mice
The mitochondrial phosphorylation capacity was decreased by 60% at day 14 post infection, and by 45% at day 19 (P < 0.01, Fig. 3D). The mitochondrial transmembrane potential was decreased by at least 40% at all time points of infection (P < 0.001, Fig. 3E). Thus, infection impaired most factors important for mitochondrial respiration, and indeed, the ATP generation ability also decreased by up to 47% (P < 0.01, Fig. 3F).
Both pro- and anti-inflammatory cytokines are expressed in vivo during C. rodentium infection
In order to identify the cytokines that may be impacting mitochondrial function we used an RT-PCR array of Th1/Th2 related genes to examine how the cytokine profile differed between day 10, 14 and 19 post C. rodentium infection. IFNγ and IL-12 mRNA were upregulated at all time points, whereas TNFα and IL-4 upregulation started at day 14 and IL-6 mRNA only increased at day 19 post infection (Table 1). The increased levels of TNFα, IFNγ and IL-12 are in line with a previous study using different time points24, and the increased levels of IL-4, TNFα and IFNγ at day 19 post infection was confirmed using individual RT-PCRs (fold increase mean [range] IL-4 5.9 [2.4–12.6], TNFα 5.3 [1.3–9.7], IFNγ 5.9 [1.8–9.6]).
Table 1. Changes in mRNA level of cytokines in wildtype and IFNγ knockout mice infected with C. rodentium.
| Cytokine | Fold changes in mRNA levels |
|||
|---|---|---|---|---|
| Day 10 wt | Day14 wt | Day19 wt | Day10 IFNγ−/− | |
| IFNγ | 8.3 | 13.7 | 72.5 | NA |
| TNFα | 1.5 | 4.7 | 11.4 | 2.5 |
| IL-12 | 2.7 | 22.7 | 42.9 | 2.2 |
| IL-4 | 1.1 | 6.9 | 4.1 | 2.8 |
| IL-6 | 2 | 2.2 | 6.5 | 20.8 |
mRNA from two sets of two mice in each group were pooled for the time points of day 0 and day 10 (i.e. data are representative of four mice in each group), whereas the time points day 14 and 19 contained mRNA pooled from three mice in each group. Data are presented as fold change compared to uninfected control mice of the same genotype. Data were normalized by the RT2 Profiler PCR Array data analysis software (QIAGEN) using the housekeeping genes Gusb, Hprt1, Hsp90ab1, Gapdh and Actb. Fold changes ≥2,5 were accepted as upregulation.
In vitro treatment with TNFα, individually and in combination with IFNγ, caused loss of complexes I and IV, which was alleviated by IL-4
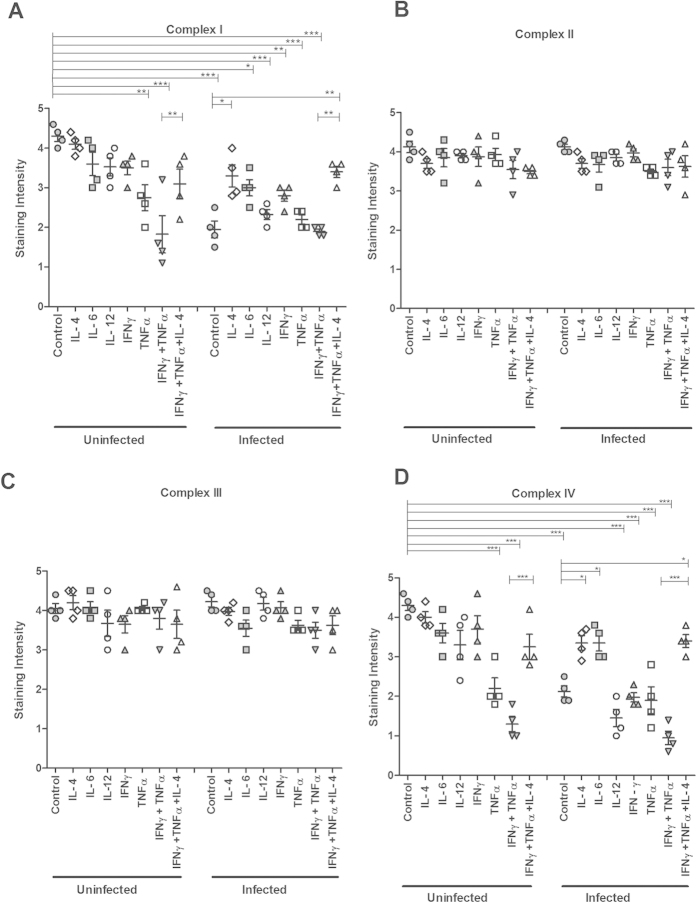
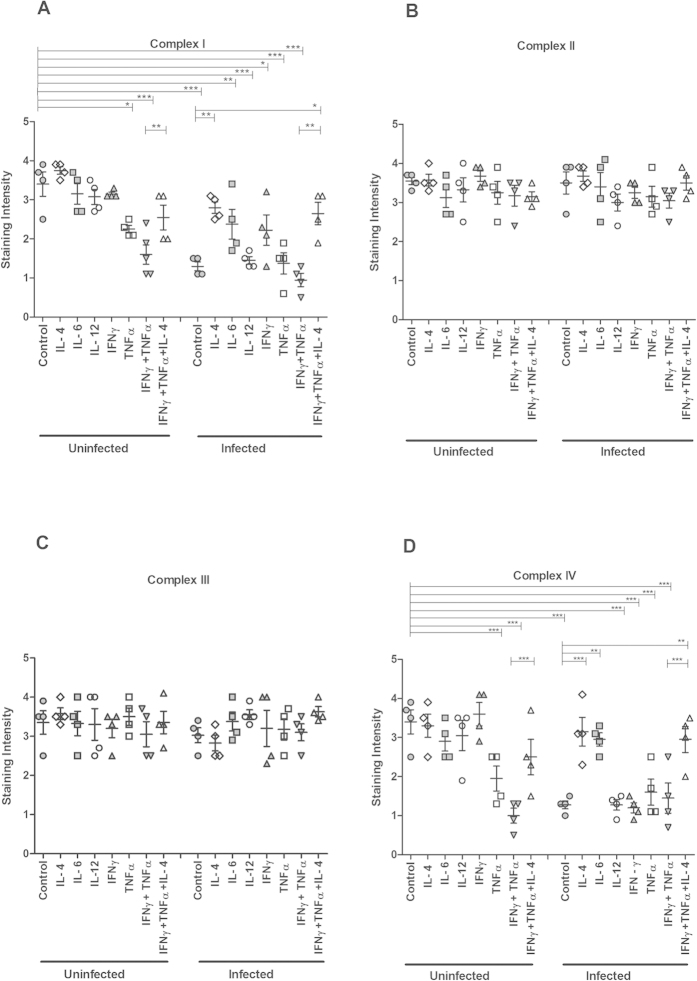
We recently developed a semi-wet interface culture method that in combination with mechanical and chemical stimulation creates an in vitro mucosal surface with polarised cells, functional tight junctions, a three-dimensional architecture and a mucus layer18. We treated this surface with cytokines for 96 h to mimic the extended period of elevated cytokine stimulus that occurred during the infection (Table 1). Immunohistochemical staining indicated that the levels of complexes I-IV remained largely unaffected by IL-4, IL-6, IL-12 and IFNγ (Fig. 4). Furthermore, no difference in intensity of complex II and complex III staining was observed in any of the other cytokine treatments performed (Fig. 4B,C). In contrast, TNFα caused a marked loss of complex-I and IV staining intensity (P < 0.01 and P < 0.001, Fig. 4A,D). Combining treatments of TNFα and IFNγ, in analogy with the in vivo cytokine expression during day 14 and 19 post infection, further decreased the intensity of the complex-I and IV staining, but this loss was alleviated by simultaneous treatment with IL-4 (P < 0.01 vs P < 0.001, Fig. 4A,D).
Figure 4. Semi-quantification of mitochondrial respiratory enzyme complexes of an in vitro intestinal model treated with cytokines and C. rodentium infection.
Immunohistochemical staining using antibodies for (A) MTND6 (complex I) (B) SDHA (complex-II) (C) CYC1 (complex-III) (D) CCO-VIc (complex-IV). Values are mean ± S.E.M. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
In vitro reduction of the protein levels of complexes I and IV caused by C. rodentium or ETEC infection was alleviated by IL-4
The transepithelial resistance of the in vitro mucosal surface remained unaffected after infection with C. rodentium (pre infection: 226 ± 22 Ω, 24 h post infection: 233 ± 23 Ω), indicating that the membranes were intact, although some bacteria had translocated across the membrane and were found in the basolateral compartment. Infection caused loss of staining for complex I and IV (P < 0.001 vs P < 0.001) but not for complex II and III (Fig. 4). IL-4 treatment reversed the infection-induced loss of staining for complex I and IV (P < 0.05, Fig. 4A,D), and IL-6 provided protection against loss of complex IV (P < 0.05, Fig. 4D). To investigate if other intestinal pathogens could have similar effects, we infected the in vitro mucosal surface with enterotoxigenic E. coli (ETEC), a human pathogen that lacks the type III secretion system and do not cause A/E lesions. ETEC infection decreased the transepithelial resistance of the in vitro mucosal membranes (pre infection: 210 ± 51 Ω, 24 h post infection: 134 ± 12 Ω), but still very similar results were obtained when ETEC was used as the infecting agent instead (Fig. 5). Together, these results indicate that infection, IFNγ and TNFα have negative effects on mitochondrial respiration, which is alleviated by IL-4, while IL-6 afforded some protection, but only against loss of complex-IV. For further mitochondrial functional studies we therefore focused on C. rodentium infection, IFNγ, TNFα and IL-4.
Figure 5. Semi-quantification of mitochondrial respiratory enzyme complexes of an in vitro intestinal model treated with cytokines and E. coli infection.
Immunohistochemical staining using antibodies for (A) MTND6 (complex I) (B) SDHA (complex-II) (C) CYC1 (complex-III) (D) CCO-VIc (complex-IV). Values are mean ± S.E.M. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
Effects on enzymatic activity of the mitochondrial respiratory complexes I and IV caused by C. rodentium infection, TNFα and IFNγ, was alleviated by IL-4
In line with the immunohistochemistry results, complex I-IV activities remained unaffected by IL-4 and IFNγ, and complex II and III activities were also not affected by TNFα and IFNγ treatments (Fig. 6). In contrast, TNFα reduced complex I and IV activity (−36% and −39%, P < 0.01, Fig. 6A,C). Combining treatments of TNFα and IFNγ, in analogy with the in vivo cytokine expression during day 14 and 19 post infection, further decreased complex I and IV activity (−58% and −52%, P < 0.001, Fig. 6A,C). IL-4 reversed the combined inhibitory impact of TNFα and IFNγ on complex I and IV activity (−58% to −28%, P < 0.01, Fig. 6A and −52% to −22%, P < 0.05, Fig. 6C).
Figure 6. Mitochondrial function of an in vitro mucosal intestinal model treated with cytokines and C. rodentium infection.
(A) complex-I activity (B) complex-II-III activity (C) complex-IV activity (D) mitochondrial phosphorylation capacity (E) mitochondrial membrane potential (F) mitochondrial ATP generation. Values are mean ± S.E.M. Statistics: ANOVA Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
Infection with C. rodentium alone led to decreases in complex I and IV activities that were counteracted by IL-4 (−58% to −13%, P < 0.05, and −55% to −14%, P < 0.001, Fig. 6A,C). Infection did not exacerbate the reduction of complex I and IV enzymatic activity caused by TNFα alone or in combination with IFNγ, and IL-4 provided similar protection against the detrimental effects of this combination in the presence of infection (from −68% to −41%, P < 0.05, and −49% to −15%, P < 0.01, Fig. 6A,C). Infection did, however, decrease the enzymatic activity of both of these complexes in cells treated with IFNγ (P < 0.01, Fig. 6A and P < 0.05, Fig. 6C). Complex II-III activity was not affected by infection with or without cytokine treatment.
In vitro, IL-4 counteracted the decreases in mitochondrial phosphorylation capacity, transmembrane potential and ATP generation caused by C. rodentium infection, TNFα and IFNγ
Reflecting the loss of complex I and IV activity, the mitochondrial phosphorylation capacity was hampered by TNFα and IFNγ both in the absence (−50%, P < 0.01) and presence (−60%, P < 0.001) of C. rodentium infection (Fig. 6D). IL-4 alleviated this impairment (P < 0.05) to a degree that it was not statistically different from non-treated mucosal membranes (Fig. 6D). Infection per se, and also in combination with TNFα and IFNγ, caused a reduction in mitochondrial phosphorylation capacity (Fig. 6D). IL-4 alleviated the impairment of the mitochondrial phosphorylation caused by the cytokines and infection, together or alone, to a degree similar to non-treated mucosal membranes (Fig. 6D). The impact of cytokines and C. rodentium infection on mitochondrial membrane potential (Fig. 6E) and ATP generation (Fig. 6F) followed a very similar pattern. Thus, IL-4 alleviated the detrimental effect of TNFα and IFNγ on ATP generation in both uninfected (from −38% to −11%, P < 0.05) and infected (from −60% to −14%, P < 0.001, Fig. 6F) conditions, and negated the direct impact of C. rodentium infection, reviving the mitochondrial ATP generation from 58% to 85% (P < 0.05).
In vivo, the levels of complex I and IV are more affected by the cytokine environment than by pathogen density
To elucidate the role of the cytokine environment versus the direct actions of C. rodentium in vivo, we studied IFNγ−/− mice, as IFNγ increased early in infection, concomitantly with the mitochondrial dysfunction (in contrast to TNFα). IFNγ−/− mice had a similar C. rodentium burden to that of the WT mice at day 10 post infection (mean ± SEM: Log 6,5 ± 0,2 CFU/g feces for IFNγ−/− and Log 6,6 ± 0,3 CFU/g feces for WT, n = 7) while at day 14 post infection the density was slightly higher in the IFNγ−/− mice (P < 0.05, Log 4,8 ± 0,3 CFU/g feces) than in the WT (mean Log 3,4 ± 0,3 CFU/g feces) mice. The cytokine environment during the course of infection was different in IFNγ−/− compared to WT mice, mainly with regards to that IL-4 and IL-6 were upregulated already by day 10 post infection (3-fold vs 20-fold, Table 1).
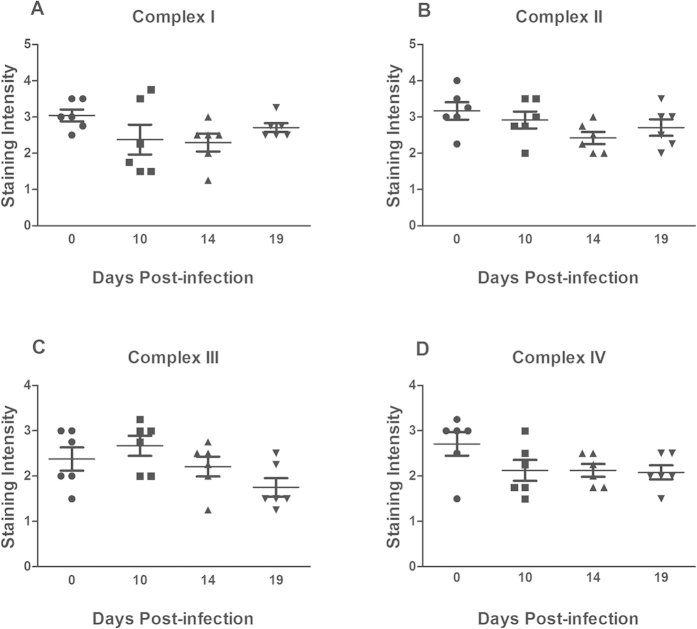
All four complexes (complex I–IV) were present relatively uniformly in the majority of the epithelial cells in the colon of IFNγ−/− mice, with a similar tissue location and staining intensity as in the WT mice (compare the non-infected controls in Figs 2 and 7). In contrast to the loss of staining intensity of subunits of complex I and IV found in colons from WT mice after infection (Fig. 2), there was no statistically significant loss of any of the four complexes in IFNγ−/− mice (Fig. 7A–D). Thus, it appears that in vivo, the cytokine environment is a more important determinant of mitochondrial complex levels than the direct actions of the pathogen. This is further supported by the observation that in the WT mice, the mitochondrial respiratory chain remained impaired even at day 19 post infection, when the pathogen burden had subsided, but the expression of TNFα and IFNγ remained elevated (Figs 2 and 3). Although the caspase-3 levels in the colonic tissue from IFNγ−/− mice were slightly elevated at day 14 post infection (p < 0.01), the magnitude of the increase was less than in the wt mice (p < 0.01, Fig. 1I), and none of the other time points showed an increase, whereas all time points in the WT animals had statistically significant increases.
Figure 7. Semi-quantitative analysis of the mitochondrial respiratory enzyme complexes in the distal colon of IFNγ−/− mice after C. rodentium infection.
Immunohistochemical staining using antibodies for (A) MTND6 (complex I) (B) SDHA (complex-II) (C) CYC1 (complex-III) (D) CCO-VIc (complex-IV). Statistics: ANOVA Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001. vs. control.
In vivo, the cytokine environment, and not the C. rodentium density, determine the level of 3-Nitrotyrosine
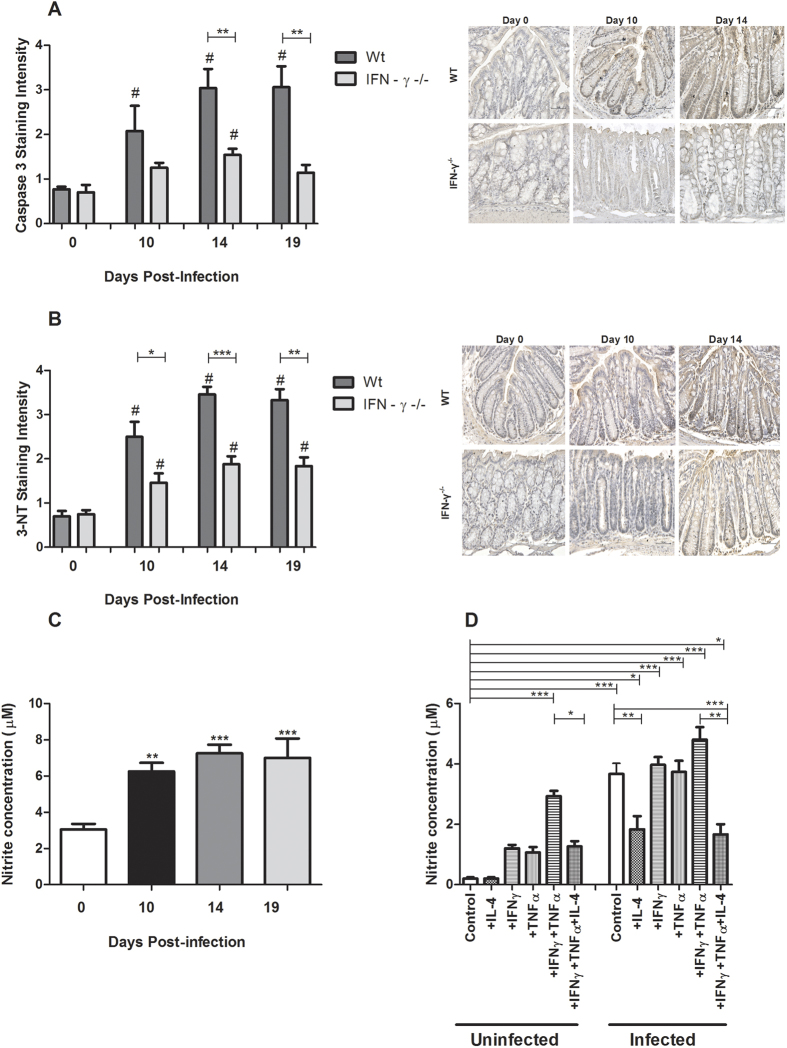
Infection with C. rodentium resulted in an increase in immunostaining intensity for 3-Nitrotyrosine (3-NT), a marker for oxidative damage, in both infected WT and IFNγ−/− mice at all time points post-infection (p < 0.05–0.0001, Fig. 8B). However, the levels of 3-NT were higher in WT mice compared to IFNγ−/− mice, with the day 14 and 19 timepoints in the WT having intensity scores twice as high as the IFNγ−/− mice (p < 0.05, Fig. 8B). Since IFNγ−/− mice had a similar pathogen burden, but a cytokine environment without IFNγ but higher in IL-4 and IL-6, this suggests that the cytokine environment, and not the pathogen burden, is the main cause of the NO generation in vivo.
Figure 8. Caspase-3, 3-NT staining scores and nitrite measurements after C. rodentium infection.
(A) The caspase-3 staining scores based on immunohistochemical staining and corresponding representative photos of the caspase-3 tissue localization in the distal colon of WT and IFNγ−/− mice. Values are mean ± S.E.M. Statistics: ANOVA with Newman-Keuls Multiple Comparison post hoc test, #P < 0.05 vs. corresponding non-infected genotype control, unpaired t test **P < 0.01 compared to WT of same infection period (n = 4–9 mice/group). Scale bar 50 μm, magnification × 400. (B) Immunohistochemical staining scores and photos representing the tissue localization of the 3-NT residues in the murine distal colons of WT and IFNγ−/− mice during infection. Scale bar 50 μm, magnification × 400. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: #P < 0.05 vs. corresponding genotype control, unpaired t test: *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT at the same time point of infection, n = 4–9 mice/group. (C) Nitrite concentration in the murine distal colon after C. rodentium infection. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001. vs. control. (D) Nitrite concentration in the in vitro mucosal intestinal model treated with cytokines and C. rodentium infection. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
In vivo and in vitro, NO generation increased during C. rodentium infection and increased levels of TNFα and IFNγ, which was counteracted by IL-4
In line with the above results, infection with C. rodentium resulted in increased generation of NO2−, another index for oxidative damage, in WT mice in all time points post-infection (P < 0.001, Fig. 8C). In the non-infected in vitro mucosal membranes, the combined action of IFNγ and TNFα led to the highest generation of NO2− (p < 0.001, Fig. 8D), and this was alleviated by IL-4 treatment (p < 0.05, Fig. 8D). In vitro infection further increased the generation of NO2−, and IL-4 alleviated both the NO2− generation-induced by the bacteria alone (p < 0.01, Fig. 8D) and by the combined actions of infection, IFNγ and TNFα (p < 0.01, Fig. 8D). Although the higher level of the NO2− generation in the infected in vitro membranes may at first glance appear to contradict the in vivo results demonstrating that the cytokine environment is a more important determinant of NO-levels than the pathogen density in vivo, these results are not surprising since in vitro, C. rodentium multiplies unhindered, and the bacterial density after 24 h of co-culture is higher than in vivo.
NO generation inversely correlated with mitochondrial function
In C. rodentium infected mice, the level of NO2− inversely correlated with complex I and complex-IV activities (Pearson product-moment correlation coefficient r2 = −0.823, p < 0.01 and r2 = −0.714, p < 0.01, respectively), mitochondrial phosphorylation (r2 = −0.846, p < 0.01), mitochondrial membrane potential (r2 = −0.735, p < 0.01) and ATP generation (r2 = −0.669, p < 0.01, compare Figs 8C and 3). Similarly, after in vitro cytokine treatment and C. rodentium infection, the level of NO2− inversely correlated with complex I and complex-IV activities (r2 = −0.851, p < 0.01 and r2 = −0.733, p < 0.01 respectively), mitochondrial phosphorylation (r2 = −0.744, p < 0.01), mitochondrial membrane potential (r2 = −0.782, p < 0.01) and ATP generation (r2 = −0.829, p < 0.01, Fig. 8D compared with Fig. 6).
In vivo and in vitro, mitoquinone (MitoQ) alleviated the damaging impact on mitochondrial function during C. rodentium infection
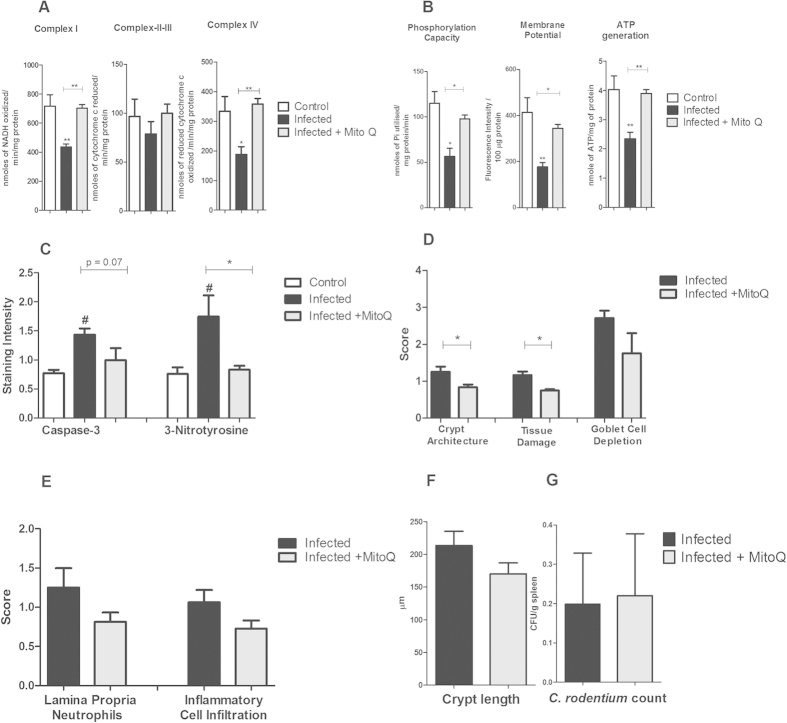
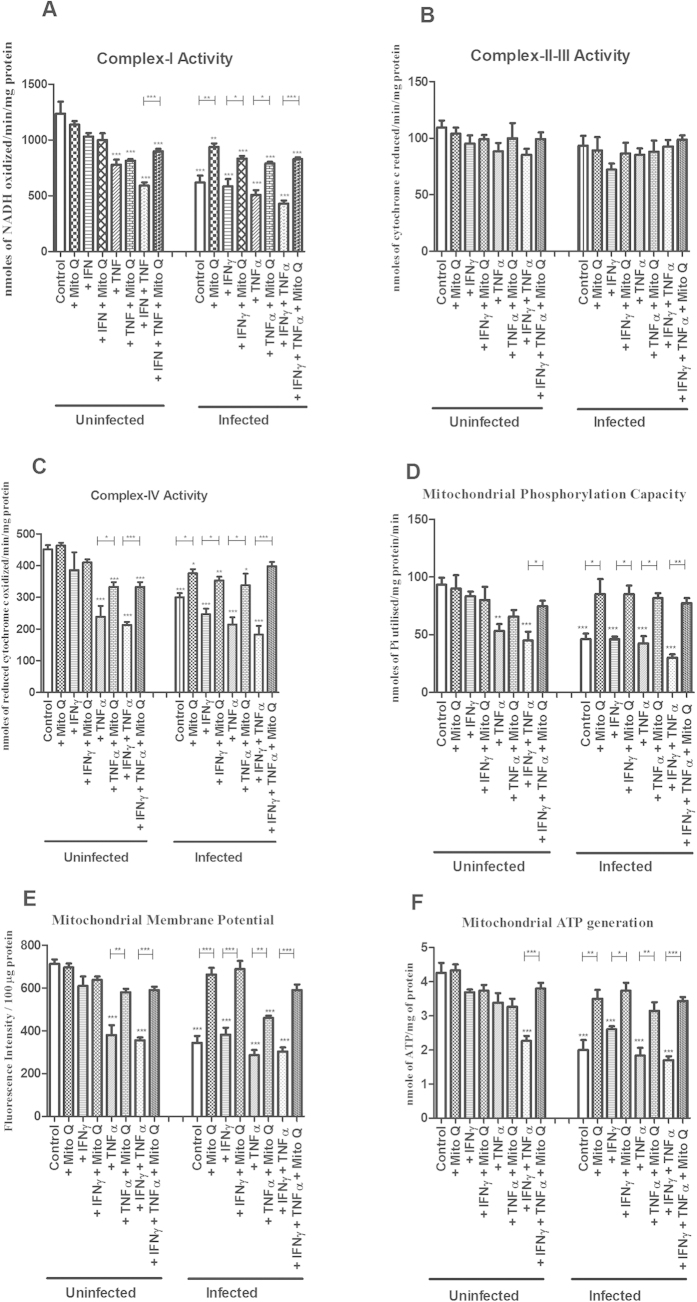
MitoQ is an antioxidant that accumulates within mitochondria, and that has been used in clinical trials in humans25. Treating mice with established infection with MitoQ (from day 5 to 14) restored the complex-I and complex-IV activities, mitochondrial phosphorylation, membrane potential and ATP generation (Fig. 9A,B). Furthermore, MitoQ treatment reverted the infection-induced 3-nitrotyrosine staining (p < 0.05, Fig. 9C). In line with these results, MitoQ also alleviated the damaging influence of infection, TNFα and IFNγ on these parameters in vitro (Fig. 10A–F).
Figure 9. Effects of MitoQ on mitochondrial functions, caspase-3, NO, colitis and C. rodentium translocation.
(A) complex I, II–III and IV activities and (B) mitochondrial phosphorylation capacity, membrane potential and mitochondrial ATP generation in infected and MitoQ treated mice. Values are mean ± S.E.M. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 (n = 4/group). (C) caspase-3 and 3-Nitrotyrosine immunostaining in infected and MitoQ treated mice. Values are mean ± S.E.M. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05 vs non-infected control, #P < 0.05 vs. infected control. (D) colonic scores of crypt architecture, tissue damage and goblet cell depletion, (E) Neutrophils in lamina propria and inflammatory cell infiltration, (F) crypt length and (G) C. rodentium counts in spleen of infected and MitoQ treated mice. Values are mean ± S.E.M. (n = 4 mice/group). Statistics: paired t test, *P < 0.05 compared to infected mice.
Figure 10. Effects of MitoQ on mitochondrial functions in the in vitro mucosal intestinal model treated cytokines with/without C. rodentium infection.
(A) complex-I activity, (B) complex-II–III activity, (C) complex-IV activity, (D) mitochondrial phosphorylation capacity, (E) mitochondrial membrane potential, and (F) mitochondrial ATP generation. Values are mean ± S.E.M. Statistics: ANOVA with Student Newman-Keuls Multiple Comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
Although treatment with MitoQ restored all of the mitochondrial parameters, not all features of the disease were improved. Crypt architecture and tissue damage improved (P < 0.05 for both, Fig. 9D), the caspase-3 levels decreased to an extent where it was not statistically different from uninfected controls, and the goblet cell depletion trended towards a decrease (p = 0.077, Fig. 9C–F). However, the number of C. rodentium in feces and spleen was similar to that of infected mice without MitoQ treatment (Fig. 9G).
Discussion
In the present study, we demonstrate for the first time that infection reduces mitochondrial complex I and IV protein levels and enzymatic activity and also phosphorylation capacity, transmembrane potential and ATP generation throughout infection and clearance of the pathogen. While previous studies have shown that bacteria can affect mitochondrial function, the endpoints examined were a loss of mitochondrial membrane potential or involvement in apoptosis induction; only one study went further and looked at complex II expression after infection13. Using an in vivo-like in vitro mucosal surface, we identified that infection per se, as well as TNFα, individually and more severely in combination with IFNγ, caused the same effects as seen in vivo. Co-treatment with IL-4, however, reversed these responses, and IL-6 also protected against loss of complex IV. The negative effects on mitochondria were caused largely by NO generation, and were reversed by mitochondrial antioxidant treatment. IFNγ−/− mice, which had a similar pathogen burden as infected WT mice, but a colonic cytokine environment with higher levels of IL-4 and IL-6, displayed no loss of any of the four complexes, demonstrating that the effects on mitochondria found in vivo were largely cytokine driven. Thus, as the concentration of IFNγ and TNFα increase during the latter time points of infection and clearance in WT mice, the concomitant increase of IL-4 and IL-6 appears to protect the mitochondrial functions of the colonic epithelium.
Of the four multimeric complexes involved in mitochondrial respiratory phosphorylation, inhibition in both levels and activities of complex I and complex IV were observed at all post-infection time points. Although the extraction process may stress the mitochondria and therefore possibly alter their function, we here provide two lines of evidence that support the results, whereof the first does not involve extraction: in situ quantification using immunohistochemistry (showing decreased levels of complex I and IV) and functional assays (demonstrating decreases in the activity of complexes I and IV, phosphorylation capacity, transmembrane potential and ATP generation). No inhibition in activity of complex II–III was noticed at any time-points. However, we did find a reduction in immunohistochemical staining intensity for complex II, which was in agreement with the earlier study13. This decrease in quantity while simultaneously retaining normal specific activity of succinate cytochrome c reductase (indicator for complex II–III activity) post-infection is puzzling. A reason for this discrepancy may be that the method adapted for measuring the activity of succinate cytochrome c reductase measures the enzymatic activity of both complex II and III, thus it is possible that normal complex III activity can mask the inhibition of complex II activity, although the method here is widely used and demonstrated to be suitable to detect complex II deficiency26. Another reason could be that a decrease in the level of enzyme can be compensated for by an increase in activity26.
Targeting of the host cell mitochondria appears to be a common strategy among many clinically important pathogens. Bacteria like Neisseria gonorrheae27, Neisseria meningitides28, Helicobacter pylori29 and Salmonella enterica serovar Typhimurium30, all target host cell mitochondria by translocating proteins that trigger cell death, mainly through apoptosis. Similarly, one report indicated involvement of C. rodentium effector proteins in causing cell death through affecting mitochondrial membrane potential and succinate dehydrogenase levels17. Cell death at the base of the colonic crypts, where the presence of C. rodentium is unlikely, has also been reported5, and our caspase-3 staining results further confirmed a similar incidence of cell death both on the luminal surface and in the crypts during infection. As cytokines have the capability to regulate epithelial cell function irrespective of the position of the cells in the crypt, we investigated the impact of cytokines that changed expression after infection, on mitochondrial function. Treatment with TNFα in vitro decreased mitochondrial functional parameters and the levels and activity of the complex I and IV enzymes, and when combined with IFNγ had an even greater effect. Even though IFNγ is known to be an immunoregulatory cytokine promoting immune responses at the initiation of several bacterial infections31,32,33,34, the impact of IFNγ on mitochondrial function during infections has not been described previously. TNFα, alone as well as together with IFNγ, has previously been shown to affect mitochondrial function in non-intestinal tissues under different experimental conditions35,36,37,38,39.
The protective effects of IL-4 on complex I and IV activity and levels that we observed in vitro is consistent with its previously observed ability to abrogate cell death by maintaining mitochondrial membrane potential and anti-oxidant status in other systems, such as B-cells40. To conclusively prove that IL-4 and IL-6 provide the same function during infection in vivo is more complicated, as changing their levels alter a whole range of infection related parameters. Indeed, IL-6 deficient mice have been shown to have high mortality and 100-fold higher pathogen burdens compared to WT mice during C. rodentium infection41. However, our results from IFNγ−/− mice supports our proposal that IL-4 and IL-6 protects the mitochondria during infection as these mice, which had higher levels of IL-4 and IL-6 but a similar pathogen burden to WT mice, had no reduction of the levels of any of the four complexes during C. rodentium infection, and greatly reduced NO generation. As loss of mitochondrial ATP generation is the consequence of dysfunction of the mitochondrial respiratory chain, these results are in line with our observation in WT animals that there are high levels of caspase-3 staining, as well as high numbers of dead and sloughed off cells during clearance of infection even at day 19 post infection, when the pathogen burden is almost entirely absent19, but the expression of IFNγ and TNFα remains high. In WT mice, when the concentrations of IFNγ and TNFα increase during clearance of infection, the concurrent induction of IL-4 and IL-6 thus appears to protect the mitochondrial function of the colonic epithelium from further damage.
Our results suggest that NO generation caused by infection-induced TNFα and IFNγ was the main cause of mitochondrial dysfunction. The dysfunction was reversed by IL-4 treatment, indicating that IL-4 has a role in regulating NO production. That the NO pathway plays a substantial role in mitochondrial dysfunction is further verified by the protective effect of the antioxidant MitoQ, which is a scavenger for the peroxynitrite (ONOO−) that is generated when NO reacts with superoxide (O2−)42. Furthermore, a previous study has shown that C. rodentium infection results in up-regulation of iNOS in the colonic epithelium in vivo, and that iNOS−/− mice are protected from C. rodentium induced inflammation, including attenuated levels of TNFα and IFNγ43. Oxidative insult to the respiratory chain complexes can amplify and promote further oxidative damage44, and indeed mitochondrial complexes I and IV, which were most effected in our study, are encoded in the mitochondrial genome45 and thereby susceptible to mutations in mitochondrial DNA.
In spite of less epithelial damage after MitoQ treatment and similar levels of C. rodentium in colon, the number of C. rodentium in the spleen did not improve. However, the number of CFU that are found in the internal organs during this infection is rather low, and possibly the ones that enter do so through mechanisms other than direct translocation across a damaged epithelial membrane. A similar mitochondria targeting antioxidant (MitoTEMPO) was recently shown to inhibit superoxide induced E. coli translocation over mucosal membranes in vitro46. To elucidate if antioxidants with protective effects towards mitochondria could have a role in treating infections, an in vivo model where bacterial translocation over the epithelium plays a more prominent role in progression of disease needs to be utilised.
In conclusion, infection with this A/E pathogen induces mitochondrial dysfunction, which is largely caused by IFNγ and TNFα synergistically compromising complex I and IV levels and activity, via NO generation. In vitro, the pathogen per se also induces a similar effect, but in vivo, the cytokine environment appears to be the dominating factor governing mitochondrial enzyme levels. The presence of both IL-4 and IL-6 during the clearance phase of infection, when TNFα and IFNγ levels are exceedingly high, protects the colonic epithelial surface against more detrimental damage.
Methods
Animals
6–8 weeks old, specific-pathogen-free, male C57BL/6 mice purchased from Taconic (England) or Charles River (Germany). IFNγ-deficient mice on a C57BL/6 background were bred and housed in ventilated cages under pathogen-free conditions at the Laboratory for Experimental Biomedicine (EBM), Sahlgrenska Academy, Gothenburg. Mice were fed ad libitum and monitored daily.
Ethics statement
All experimental procedures were approved, and performed in accordance with, the guidelines laid by the Göteborgs Djurförsöksetiska Nämnd (Ethic No. 261/09) based on regulations from Djurskyddsförordningen DFS 2004:4.
Culture of the in vitro colonic mucosal model
For propagation, the human intestinal cell line HT29 MTX-E12 was cultured (at 37 °C, 5% CO2 −95% air) in RPMI containing 10% (v/v) FCS, 1% 5000 U (v/v) penicillin-streptomycin (Lonza). To form the in vitro colonic mucosal surface18, 7.5 × 104 cells in 200 μl of RPMI containing 10% (v/v) FCS and penicillin-streptomycin were added to the apical side of Snapwell membranes (0.4 mm pores) with 12 mm diameter (Corning). When cells became confluent (4–6 days later) they were subjected to semi-wet interface culture with continuous rocking, with 2 ml media in the basolateral compartment and 50 μl of media in the apical compartment for 28 days. Basolateral media was refreshed every two days and for the first 6 days it was supplemented with 10 mM N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester (DAPT, Sigma-Aldrich).
Infection and treatments
Mice: C. rodentium strain ICC169 was grown on MacConkey agar (Oxoid) for 20 h at 37 °C. Male C57BL/6 wild type and IFNγ−/− mice were infected with 100 μl of bacterial suspension (5 × 109 colony forming units (CFU) in Luria-Bertani (LB) broth) by oral gavage. Infection experiments were performed twice for each time point. MitoQ treatment: infected mice were administered MitoQ orally through drinking water (500 μM) from day 5 to 14 of infection period. This dose of MitoQ was chosen as it was not toxic in an earlier study47. Mice were anaesthetised with isoflurane and killed by cervical dislocation at day 10, 14 and 19 post infection. The last 2.5 cm of colon, beginning at the anal verge, was collected. For the first set of experiments for all time points, the most distal 1 cm were harvested into fresh Carnoy’s fixative (60% dry methanol, 30% chloroform, 10% glacial acetic acid); the next distal 1 cm colonic specimens stored in RNAlater (Ambion) for RNA isolation. For the second set of experiments, the most distal 1.5 cm colonic specimens were harvested into Carnoy’s methanol fixative and the next distal 1 cm colonic specimens in ice cold imidazole buffer (50 mM, pH 7.4) for mitochondrial isolation.
In vitro cytokine treatments and infection: In vitro mucosal surfaces (described above) were treated with cytokines for 96 h, starting on day 28 post confluency. The 96 h duration treatment was to mimic physiological conditions during infection, as colon epithelial cells were exposed to elevated cytokine levels for days (table 1). The cultures were exposed individually and in combination with IFNγ (10 ng/ml), TNFα (10 ng/ml), IL-4 (1.5 ng/ml), IL-6 (15 ng/ml) and IL-12 (20 ng/ml); these concentrations were based on previous work41,48,49,50,51,52,53. For MitoQ treatment, 50 nM was added to the basolateral side for 96 h, the dose was based on previous work47. Antibiotic-free RPMI containing 10% FBS, cytokines and/or MitoQ was changed every 24 h54,55,56. For infection, 10 μl of C. rodentium and enterotoxigenic E. coli (ETEC, strain E2265) suspensions with a respective OD of 2.0 and 0.1 at 410 nm (CFU: 107 and 5 × 105 CFU, corresponding to a multiplicity of infection of 10:1 and 0.5:1 ) in sterile PBS was added to the apical side of the membrane 24 h prior to the experimental end point. Although the epithelial surface is exposed to bacteria for days in vivo, this was not technically possible in vitro, due to overgrowth of the bacteria. To monitor the effects of infection on the membranes, Trans Epithelial Electrical Resistance (TEER) was measured using an EVOM2 meter and STX2 probe (World Precision Instruments, Sarasota, Florida, USA).
Histology-For colitis analysis in infected mice, 5 μm sections of Carnoy’s fixed tissue were stained with haematoxylin/eosin, coded to blind the analysis, and the entire section was systematically scored for: aberrant crypt architecture (0–3), tissue damage (0–3), increased crypt length (0–3), goblet cell depletion (0–3, confirmed by PAS/Alcian blue stain18), lamina propria neutrophil counts (0–3), crypt abscesses (0–3) and inflammatory cell infiltration (0–3).
RT-PCR for cytokines
For the quantitative RT-PCR cytokine array, total RNA was extracted from distal colon using the RNeasy mini kit (QIAGEN), and cDNA prepared using the QuantiTect Reverse Transcription kit (QIAGEN). mRNA from two sets of two mice in each group were pooled for the time points of day 0 and day 10 (i.e. data representative of four mice in each group), while time points day 14 and 19 contained mRNA pooled from three mice in each group. RT-PCR on pooled samples was carried out in duplicate using RT2 Profiler™ PCR Array (PAMM-034Z) plates containing 84 mouse inflammatory cytokine, chemokine and receptor genes (QIAGEN). The arrays were run on an ABI 7500 real-time PCR system (Applied Biosystems). Intra-plate controls were included and data were normalised by the RT2 Profiler PCR Array data analysis software (QIAGEN) using the most suitable housekeeping gene chosen from five housekeeping genes [Gusb (Glucuronidase, beta), Hprt1 (Hypoxanthine guanine phosphoribosyl transferase1), Hsp90ab1 (Heat shock protein 90 kDA alpha (cytosolic), class B member 2), Gapdh (Glyceraldehyde-3-phosphate dehydrogenase), Actb (Actin, beta cytoplasmic)] present in the plates, with a threshold of variance of 0.2 cycles. Fold changes against control mice were calculated using the same software. Fold changes ≥2.5 were accepted as up- or downregulation. For the individual RT-PCRs, total RNA was extracted from three uninfected WT control mice and three mice from day 19 post infection using TRizol (Life Technologies, Carlsbad, CA, USA). RNA purity was assessed through UV spectroscopy (NanoDrop; Thermo Scientific, MA, USA). Total RNA (5 μg) was treated with DNase at 37 °C for 30 min, addition of 5 mM EDTA and heat inactivation of DNase at 75 °C for 10 min followed by cDNA synthesis. A final concentration of 5 mM MgCl2 was added to RNA samples, which were later used for cDNA synthesis by adding oligo-dT primers and Superscript III (Life Technologies, Carlsbad, CA, USA) at 50 °C for 2 h. The cDNA was used in a RT-PCR using Evagreen SSO-Fast (Bio-Rad laboratories, Hercules, CA, USA) and IL-4 (Fwd: GGCTTTTCGATGCCTGGATT, Rev: TTTGCATGATGCTCTTTAGGCTTT), TNFα57 and IFNγ primers57. The expression of hprt1 (QIAGEN) and eif2 (Eukaryotic initiation factor 2) (designed using Primer3 program http://frodo.wi.mit.edu/primer3/ Fwd:GCTTCCCTGTTCACCTCTGA, Rev: CACATGGGCGATGACTGAC) reference genes were used for normalizing the qPCR data. Samples were amplified in triplicate with a negative control without reverse transcriptase to confirm the lack of contaminating genomic DNA. Data acquisition and analysis were performed using CFX manager 3.1 software (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Immunohistochemistry
Antigen retrieval was performed using Dako target retrieval solution, pH 9.0 (S2367, Dako) 30 min (murine samples) or 15 min (in vitro mucosal surface) at 95 °C then cooled in room temperature for 40 min. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide in PBS, 15 min and non-specific antibody binding prevented by blocking in Dako protein block serum-free reagent (X0909, Dako) for 30 min, room temperature prior to incubation with primary antibodies. Rabbit polyclonal primary antibodies for subunits of the mitochondrial respiratory enzyme complexes were selected for complex I (anti-NADH dehydrogenase subunit 6, MTND6, orb6548, Biorbyt), complex-II (anti-Succinate dehydrogenase subunit A, SDHA, ab86932, Abcam), complex-III (anti-Ubiquinol-cytochrome c reductase complex cytochrome c1 subunit, CYC1, NBP1-86872, Novus Biologicals), complex-IV (anti-Cytochrome c oxidase subunit VIc ,CCO-VIc, ab150422, Abcam), Caspase-3 (activated form, ab4051, Abcam) and 3-Nitrotyrosine (AB5411, Millipore) at optimal dilutions of 1:2000, 1:200, 1:2000, 1:2000, 1:500 and 1:500 respectively, in antibody diluent (Dako, S0809). Incubation with the complex-II (SDHA) and Caspase-3 primary antibodies was performed overnight at 4 °C; incubation with complex I, III, IV and 3-Nitrotyrosine antibodies were for 3 h at 23 °C. Sections were then washed four times in PBS containing 0.05% Tween 20, then incubated with a horseradish peroxidase (HRP) labelled anti-rabbit polymer for 15 min using a reagent kit from Dianova GmbH (PT03-L). Immunostaining was visualised using 0.05% 3,3′-diaminobenzidine hydrochloride (DAB) chromogen (CD-12, Dako) for 10 min, then counterstained with haematoxylin. Scoring of staining intensity was performed blinded, and scores of (blinded) key samples were verified by a second independent observer. Scores represent the average of 1 cm distal colon, using a scale of 0–5.
Isolation of the mitochondrial fraction
Murine colon: Specimens collected in ice cold imidazole buffer (50 mM, pH 7.4) were scraped to collect epithelial cells in a petri dish on ice. Epithelial scrapings were suspended in 5 ml of homogenizing buffer A [225 mM mannitol, 75 mM sucrose, 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mg/ml bovine serum albumin (BSA, pH 7.4)] and homogenised with a tissue homogenizer (VWR-VDI12,VWR) using 7–10 strokes. The homogenate was brought to 15 ml with the same buffer and centrifuged at 1000 g for 10 min at 4 °C. The supernatant was saved, the pellet was resuspended in homogenizing buffer A and centrifuged again at 1000 g for 10 min at 4 °C. Supernatants from these two steps were pooled and centrifuged at 10000 g for 10 min at 4 °C. The supernatant was stored at −20 °C for the nitrite assay and the pellet was utilised for mitochondrial isolation. The pellet was resuspended in homogenization buffer A without EGTA and BSA, then centrifuged at 10000 g for 10 min at 4 °C. For measurement of mitochondrial respiratory complex activities, mitochondria were resuspended in 50 mM phosphate buffer, pH 7.4; for measurement of membrane potential, mitochondrial ATP and phosphorylation capacity, the final mitochondrial pellet was resuspended in an isotonic buffer (145 mM KCl, 50 mM sucrose, 1 mM EGTA, 1 mM magnesium chloride, 10 mM phosphate buffer, pH 7.4). All aliquots of mitochondrial suspensions were kept frozen at −20 °C and used within a week.
In vitro mucosal surface
The method of Modica-Napolitano et al. 198958 with minor modifications was used: Cells from 12 membranes, pooled into 3 replicates (4 membranes per sample) per combination of treatment regimen were harvested in culture medium, pelleted, and washed with homogenization buffer B (250 mM sucrose, 1 mM Tris-HCI, l mM ethylene diaminetetraacetic acid (EDTA, Sigma-Aldrich), 1 mg/ml of BSA, pH 7.4) at 4 °C. The cells were resuspended in 5 ml of ice-cold homogenization buffer B, homogenised with 110 strokes to disrupt at least 95% of the cells58, centrifuged at 1000 g for 10 min at 4 °C. The supernatant was saved, the pellet resuspended and centrifuged again at 1000 g for 10 min at 4 °C. The supernatants from these two steps were pooled and centrifuged at 10000 g for 10 min. The supernatant obtained was stored at −20 °C for the nitrite assay and the pellet resuspended in homogenization B buffer containing digitonin (0.02%), then centrifuged again at 10000 g, and finally resuspended in an appropriate buffer for further experimentation (same as for mitochondria from colon tissue, above).
Assessing mitochondrial respiratory enzyme complex activities
The complex activities in the mitochondrial fractions were measured following standard protocols: NADH-ferricyanide reductase (complex-I) activity was measured following the method of Hatefi59 using ferricyanide as the electron acceptor in a system containing 0.17 mM NADH, 0.6 mM ferricyanide, and Triton X-100 (0.1% v/v) in 50 mM phosphate buffer, pH 7.4 at 30 °C. The reaction was initiated with addition of the mitochondrial suspension (10 μg protein), and the rate of oxidation of NADH was measured by the decrease in absorbance at 340 nm.
The activity of succinate cytochrome c reductase (complex II–III) was assayed by following the succinate supported reduction of ferricytochrome c to ferrocytochrome c at 550 nm in an assay mixture containing 100 mM phosphate buffer, 2 mM succinate, 1 mM KCN, 0.3 mM EDTA and 1.2 mg/ml cytochrome c (Sigma-Aldrich) in a total volume of 0.4 ml60. The reaction was initiated by adding mitochondrial suspension (10 μg protein) to the sample cuvette. The same assay was repeated with (10 μM) antimycin (Sigma-Aldrich) to determine the inhibitor sensitive rate and the results were expressed as nmoles of cytochrome c reduced/min/mg protein.
The activity of cytochrome c oxidase (complex IV) was assayed by measuring the rate of decrease of absorbance at 550 nm at room temperature following the oxidation of reduced cytochrome c (50 μM) in 10 mM phosphate buffer, pH 7.461. Ferricyanide (1 mM) was added to oxidize ferrocytochrome c in the blank cuvette and the reaction initiated in the sample cuvette by the addition of mitochondrial suspension (10 μg). The activity of the enzyme was expressed as nmoles of cytochrome c oxidised/min/mg protein.
Measurement of mitochondrial phosphorylation capacity
Phosphate utilization was assayed following a method published previously62. In a total volume of 250 μl, an aliquot of 25 μl mitochondrial suspension was diluted into a medium containing 125 mM KCl, 75 mM sucrose, 0.1 mM EGTA, 1 mM MgCl2, 10 mM HEPES, 2 mM phosphate, 0.3% BSA, 0.5 mM ADP (Sigma-Aldrich), 5 mM pyruvate, 10 mM succinate and 10 mM glucose, followed by immediate addition of 5 units of hexokinase (Sigma-Aldrich) and incubation at 37 °C for 30 min. The reaction was terminated by addition of 5% ice cold trichloroacetic acid (TCA) and the amount of inorganic phosphate was measured spectrophotometrically. A 0 min sample was also assayed for inorganic phosphate content where hexokinase addition was immediately followed by treatment with 5% ice cold TCA. Glucose and hexokinase in the reaction mixture acted as a trap for ATP to maintain the level of ADP in the system and also to prevent the release of free inorganic phosphate from ATP by the action of various phosphatases.
ATP synthesis
ATP content was measured in aliquots of mitochondrial suspension by a colorimetric assay method using the phosphorylation of glycerol to generate a quantifiable product at 570 nm using a commercial ATP assay kit (ab83355, Abcam).
Measurement of mitochondrial transmembrane potential
Murine colon: aliquots of mitochondrial suspensions were incubated at 37 °C for 30 min in isotonic buffer A containing 10 mM pyruvate, 10 mM succinate and 1 mM ADP with 5 μM JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′- tetraethyl benzimidazolylcarbocyanine iodide, CS0760, Sigma-Aldrich). After incubation, the dyed mitochondria were collected by centrifugation, washed with isotonic buffer A to remove excess dye and resuspended in the same buffer in appropriate dilution, followed by measurement of fluorescence intensity (λex 490 nm, λem 590 nm). In vitro mucosal surface: Mitochondrial transmembrane potential in intact HT29 MTX-E12 cells was measured using a tetramethylrhodamine ethyl ester (TMRE) mitochondrial membrane potential assay kit (ab113852, Abcam) following the manufacturer’s instructions.
Measurement of citrate synthase activity
The activity was determined spectrophotometrically at 30 °C according to the method of Srere (1969)63. The assay medium consisted of 0.1 M tris-HCl (pH-8.5), 0.1 mM 5-dithiobis-2- nitrobenzoic acid (DTNB), acetyl CoA, 500 μM oxaloacetic acid, and a mitochondrial suspension (10 μg protein) in a total volume of 1 ml. As citrate synthase irreversibly catalyzes the reaction CoA-SH + DTNB → TNB + CoA-S-S-TNB, the readout product used was thionitrobenzoic acid (TNB) which can be quantified by absorption at 412 nm. One unit of enzyme is defined as 1 μmol of oxaloacetate utilized/min per mg of protein.
Measurement of total nitrite release
Nitrite levels were determined in the cell free supernatant obtained from cell and tissue homogenate (see mitochondria isolation section). Nitrate reductase (ab156629, Abcam) was used to convert nitrates to nitrites and the rest of the procedure was adapted from the Griess reagent kit (G-7921, Life Technologies).
Protein concentration
Estimated after solubilizing the samples in 1% SDS following the method of Lowry64.
Statistical analysis
All tests were performed using Prism (GraphPad Software, version 3·0) or SPSS statistics 18 (IBM). Values are expressed as mean ± S.E.M. Comparison of data between control and infected at a specific time-point was made using the unpaired t test. Differences were considered significantly different if P was <0.05. One-way analysis of variance (ANOVA) with Student Newman-Keuls Multiple Comparison test was used to compare data for more than 2 experimental groups. Normality was confirmed using the Kolmogorov-Smirnoff test, and homoscedasticity was confirmed using the Bartlett’s test. Only one data set failed the test (Fig. 2D), but passed these tests after Log10 transformation. For Figs 5 and 9, the number of n in each treatment group was too small (n = 3) to perform these tests, however, overall the data followed a normal distribution, and in experiments with several treatments and balanced data, heterogeneous variances do not noticeably increase the risk of Type I error65. All aspects considered, we decided One-Way ANOVA followed by post hoc testing using Student Newman-Keuls test was the most appropriate way to treat the data since One-Way ANOVA is a robust statistical test if sample sizes are similar. The Pearson product-moment correlation coefficient was used for analyzing correlations.
Additional Information
How to cite this article: Maiti, A. K. et al. IL-4 Protects the Mitochondria Against TNFa and IFNγ Induced Insult During Clearance of Infection with Citrobacter rodentium and Escherichia coli. Sci. Rep. 5, 15434; doi: 10.1038/srep15434 (2015).
Acknowledgments
We thank Prof. C. Gustafsson and Drs E. Skoog, M. Holmström and J Asin Cayuela for reviewing the manuscript prior to submission. This work was supported by the Swedish research council [521-2011-2370], the Swedish Research Counsil Formas [221-2011-1036 and 221-2013-590] and the Novo Nordisk, Åke Wibergs, Lundgrens and Söderbergs Foundations. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions A.K.M., N.N., S.S. and H.F. performed the experiments. A.K.M. and S.K.L. designed them and A.K.M., S.S. and S.K.L. interpreted the data. A.K.M., H.R.F. and S.K.L. wrote the manuscript, and all authors reviewed it.
References
- Barnett F. D., Abul-Milh M., Huesca M. & Lingwood C. A. Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect. Immun. 68(6), 3108–3115 (20l0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Li Y., Vallance B. A. & Finlay B. B. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69(10), 6323–6335 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel G. et al. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 64, 5315–5325 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luperchio S. A. & Schauer D. B. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3, 333–340 (2001). [DOI] [PubMed] [Google Scholar]
- Vallance B. A., Deng W., Jacobson K. & Finlay B. B. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 71, 3443–3453 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P. M., Olmstead L. & Petras R. E. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology. 99(1), 142–149 (1990). [DOI] [PubMed] [Google Scholar]
- Crane J. K., Majumdar S. & Pickhardt D. F. 3rd. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 67(5), 2575–2584 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Zamzami N. & Susin S. A. Mitochondrial control of apoptosis. Immunology today 18, 44–51 (1997). [DOI] [PubMed] [Google Scholar]
- Desagher S. & Martinou J. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10(9), 369–377 (2000). [DOI] [PubMed] [Google Scholar]
- Dean P., Maresca M. & Kenny B. EPEC’s weapons of mass subversion. Curr. Opin. Microbiol. 8, 28–34 (2005). [DOI] [PubMed] [Google Scholar]
- Deng W. et al. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci USA. 101(10), 3597–3602 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer D. B. & Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect. Immun. 61(6), 2486–2492 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C. et al. Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map). Cell Microbiol. 8(10), 1669–1686 (2006). [DOI] [PubMed] [Google Scholar]
- Mundy R., MacDonald T., Dougan G., Frankel G. & Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 7(12), 1697–1706 (2005). [DOI] [PubMed] [Google Scholar]
- Nougayrède J. P. & Donnenberg M. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol. 6(11), 1097–1111 (2004). [DOI] [PubMed] [Google Scholar]
- Nagai T., Abe A. & Sasakawa C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280, 2998–3011 (2005). [DOI] [PubMed] [Google Scholar]
- Lopez-Armada M. J., Riveiro-Naveira R. R., Vaamonde-Garcia C. & Valcarcel-Ares M. N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13, 106–118 (2013). [DOI] [PubMed] [Google Scholar]
- Navabi N., McGuckin M. & Lindén S. K. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS One. 8(7), 68761 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J. K. et al. Dynamic changes in mucus thickness and ion secretion during Citrobacter rodentium infection and clearance. PloS one 8(12), e84430 10.1371/journal.pone.0084430 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula S. M. & Ashwell J. D. IAPs: what’s in a name? Mol. cell 30, 123–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright K. M. & Salvesen G. S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15, 725–731 (2003). [DOI] [PubMed] [Google Scholar]
- Florey H. W.. Electron microscopic observations on goblet cells of the rat’s colon. Q. J. Exp. Physiol. Cogn. Med. Sci. 45 329–336 (1960). [DOI] [PubMed] [Google Scholar]
- Ding W. X. & Yin X. M. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 393(7), 547–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins L. M., Frankel G., Douce G., Dougan G. & MacDonald T. T. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 67(6), 3031–3039 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A. & Murphy M. P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad. Sci. 1201, 96–103 (2010). [DOI] [PubMed] [Google Scholar]
- Taylor R. W., Birch-Machin M. A., Bartlett K. & Turnbull D. M. Succinate-cytochrome c reductase: assessment of its value in the investigation of defects of the respiratory chain. Biochimica et biophysica Acta 1181, 261–265 (1993). [DOI] [PubMed] [Google Scholar]
- Muller A. et al. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. The EMBO journal 19, 5332–5343 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari P., King C., Ho A. Y. & Wetzler L. M. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 5(2), 99–109 (2003). [DOI] [PubMed] [Google Scholar]
- Galmiche A. et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. The EMBO journal 19, 6361–6370 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L. D., Pypaert M., Flavell R. A. & Galan J. E. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 163, 1123–1131 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T. et al. Interleukin 12-dependent interferon gamma production by CD8alpha + lymphoid dendritic cells. J. Exp. Med 189, 1981–1986 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi H. et al. Gamma interferon produced by antigen-specific CD4 + T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect. Immun. 78, 2653–2666 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D. K. et al. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259, 1739–1742 (1993). [DOI] [PubMed] [Google Scholar]
- Schroder K., Hertzog P. J., Ravasi T. & Hume D. A. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of leukocyte biology 75, 163–189 (2004). [DOI] [PubMed] [Google Scholar]
- Busquets S. et al. Tumour necrosis factor-alpha uncouples respiration in isolated rat mitochondria. Cytokine 22, 1–4 (2003). [DOI] [PubMed] [Google Scholar]
- Lancaster J. R. Jr., Laster S. M. & Gooding L. R. Inhibition of target cell mitochondrial electron transfer by tumor necrosis factor. FEBS letters 248, 169–174 (1989). [DOI] [PubMed] [Google Scholar]
- Ledgerwood E. C. et al. Tumor necrosis factor is delivered to mitochondria where a tumor necrosis factor-binding protein is localized. Lab. Invest. 78(12), 1583–1589 (1998). [PubMed] [Google Scholar]
- Geng Y., Hansson G. & Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ. Res. 71(5), 1268–1276 (1992). [DOI] [PubMed] [Google Scholar]
- Kamachi M. et al. Regulation of apoptotic cell death by cytokines in a human salivary gland cell line: distinct and synergistic mechanisms in apoptosis induced by tumor necrosis factor alpha and interferon gamma. J. Lab. Clin. Med. 139(1), 13–19 (2002). [DOI] [PubMed] [Google Scholar]
- Lemaire C., Andr Au, K., Fraisse C. S., Adam A. & Souvannavong V. IL-4 inhibits apoptosis and prevents mitochondrial damage without inducing the switch to necrosis observed with caspase inhibitors. Cell Death Differ. 6(8), 813–820 (1999). [DOI] [PubMed] [Google Scholar]
- Dann S. M. et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 180, 6816–6826 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. M., Cocheme H., Smith R. A. & Murphy M. P. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol. Chem. 280(22), 21295–21312 (2005). [DOI] [PubMed] [Google Scholar]
- Gobert A. P. et al. Protective role of arginase in a mouse model of colitis. J Immunol. 173(3), 2109–17 (2004). [DOI] [PubMed] [Google Scholar]
- Kowaltowski A. J. & Vercesi A. E. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 26, 463–471 (1999). [DOI] [PubMed] [Google Scholar]
- Schon E. A., DiMauro S. & Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat.Rev. Genet. 13, 878–890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am. J. Pathol. 184, 2516–2527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashdorj A. et al. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC medicine 11, 178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zund G., Madara J. L., Dzus A. L., Awtrey C. S. & Colgan S. P. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J.Biol.Chem. 271, 7460–7464 (1996). [DOI] [PubMed] [Google Scholar]
- Smirnova M. G., Kiselev S. L., Birchall J. P. & Pearson J. P. Up-regulation of mucin secretion in HT29-MTX cells by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-6. Eur Cytokine Netw 12, 119–125 (2001). [PubMed] [Google Scholar]
- Hiscox S., Hallett M. B., Puntis M. C. & Jiang W. G. Inhibition of cancer cell motility and invasion by interleukin-12. Clin. Exp. Metastasis 13, 396–404 (1995). [DOI] [PubMed] [Google Scholar]
- Adams R. B., Planchon S. M. & Roche J. K. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J. Immunol. 150, 2356–2363 (1993). [PubMed] [Google Scholar]
- Harrop C. A., Gore R. B., Evans C. M., Thornton D. J. & Herrick S. E. TGF-beta(2) decreases baseline and IL-13-stimulated mucin production by primary human bronchial epithelial cells. Exp. Lung Res. 39, 39–47 (2013). [DOI] [PubMed] [Google Scholar]
- Treede I. et al. TNF-alpha-induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidylcholine in intestinal epithelial cells. BMC gastroenterology 9, 53 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarant J. M. Blood cytokines as biomarkers of in vivo toxicity in preclinical safety assessment: considerations for their use. Toxicol Sci. 117(1), 4–16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa N. et al. Prolonged circulation half-life of interferon gamma activity by gene delivery of interferon gamma-serum albumin fusion protein in mice. J. Pharm. Sci. 100, 2350–2357 (2011). [DOI] [PubMed] [Google Scholar]
- Finkelman F. D. & Morris S. C. Development of an assay to measure in vivo cytokine production in the mouse. Int. Immunol. 11, 1811–1818 (1999). [DOI] [PubMed] [Google Scholar]
- Flach C. F., Ostberg A. K., Nilsson A. T., Malefyt Rde W. & Raghavan S. Proinflammatory cytokine gene expression in the stomach correlates with vaccine-induced protection against Helicobacter pylori infection in mice: an important role for interleukin-17 during the effector phase. Infect Immun 79, 879–886 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Steele G. D. Jr. & Chen L. B. Aberrant mitochondria in two human colon carcinoma cell lines. Cancer Res. 49, 3369–3373 (1989). [PubMed] [Google Scholar]
- Hatefi Y. Preparation and properties of NADH: ubiquinone oxidoreductase (complexI), EC 1.6.5.3. Methods Enzymol. 53, 11–14 (1978). [DOI] [PubMed] [Google Scholar]
- Wharton D. C. & Tzagoloff A. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 10, 245–250 (1967). [Google Scholar]
- Clark J. B., Bates T. E., Boakye P., Kuimov A. & Land J. M. Investigation of mitochondrial defects in brain and skeletal muscle. In: Neurochemistry: A Practical Approach (Turner A. J. & Bachelard H. S. Eds.) pp. 151–174, Oxford University Press, Oxford (1997). [Google Scholar]
- Hinkle P. C. Oxygen proton and phosphate fluxes and stoichiometries. in: Bioenergetics, A Practical Approach (Brown G. C. & Cooper C. E. Eds.) pp 1–15 IRL Press, Oxford (1995). [Google Scholar]
- Srere P. A. Citrate Synthase. Methods Enzymol. 13, 3–11 (1969). [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L. & Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951). [PubMed] [Google Scholar]
- Underwood A. J. Environmental decision-making and the precautionary principle: What does this principle mean in environmental sampling practice? Landscape Urban Plan 37, 137–146 (1997). [Google Scholar]