Figure 4.
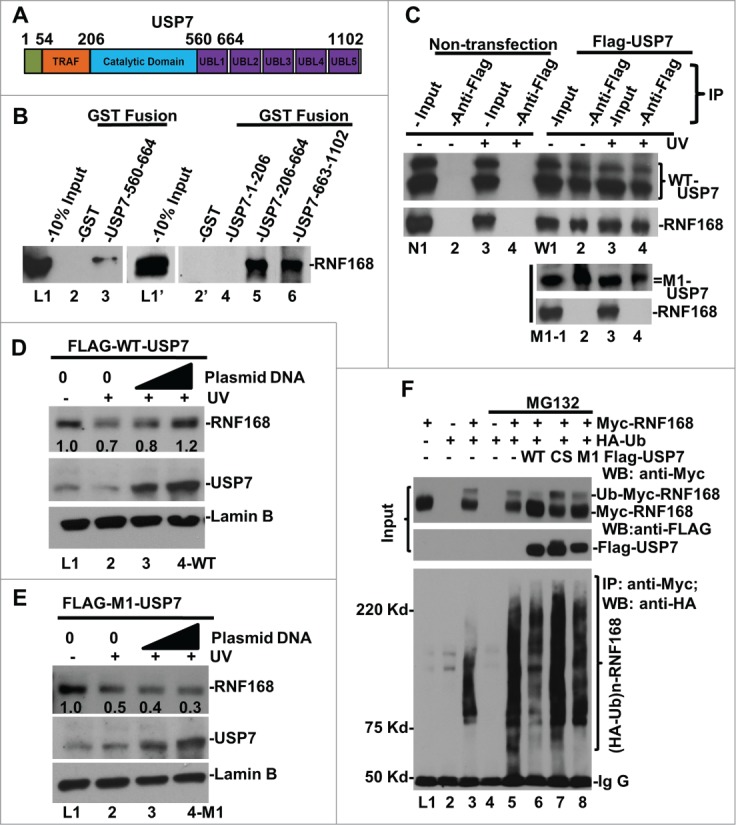
USP7 binds to RNF168, deubiquitinates RNF168 ubiquitin (Ub)-conjugates and protects RNF168 from UV-induced degradation. (A) Diagram of structural domains of USP7. TRAF represents tumor necrosis factor-receptor associated factor (TRAF) domain. UBL represents Ub-like domain. (B) USP7 interacts with RNF168 in vitro. GST pull-down assay were conducted using whole cell lysates of HCT 116 in RIPA buffer. The GST fusion proteins loaded on beads were verified as described in materials and methods. (C) Interaction between RNF168 and USP7 in vivo. FLAG-tagged USP7 and M1 mutant were transiently expressed in HCT116 cells. Immunoprecipitation was performed using anti-FLAG agarose gels and cells lysates made in E1A buffer, followed by Western blot analysis for presence of RNF168. Non-transfected HCT116 cells were used as control. (D) Overexpression of WT-USP7 protects RNF168 from UV-induced degradation. Construct for expressing FLAG-tagged USP7 was transfected into HCT116 cells for 48 h. The transfected cells were UV irradiated at 20 J/m2 and allowed to repair DNA for 2 h. RNF168 and USP7 were detected by Western blotting. Anti-Lamin B blots served as loading control. (E) Overexpression of M1 mutant USP7. (G) USP7 deubiquitinates RNF168 Ub-conjugates in vivo. HCT116 cells were transfected with expressing constructs for RNF168, HA-tagged Ub and FLAG-tagged USP7 in combination as illustrated in the figure. The transfected cells were treated with proteasome inhibitor MG132 or vehicle DMSO for 8 h. Expression of transfected RNF168 and USP7 was examined by Western blotting (Input); RNF168 Ub-conjugates were examined by immunoprecipitation followed by Western blotting.
