Figure 10. Schematic representation of the effect of Ser250 phosphorylation on SDS molecule binding.
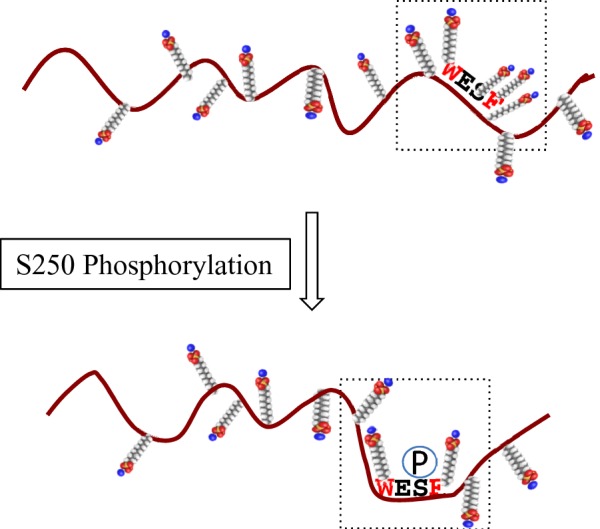
Phosphorylation of Ser250, which is located in a disordered region and surrounded by hydrophobic tryptophan and phenylalanine residues, changes the electrostatic environment by reducing the number of SDS molecules bound in the local SDS/protein complex, resulting in a mobility shift on SDS/PAGE. The small molecules represent SDS with the sulfates shown in red.
