Abstract
Diagnosis and management of coronary artery disease represent a major challenge to our health care systems affecting millions of patients each year. Until recently, the diagnosis of coronary artery disease could be conclusively determined only by invasive coronary angiography. To avoid risks from cardiac catheterization, many healthcare systems relied on stress testing as gatekeeper for coronary angiography. Advancements in cardiac computed tomography angiography technology now allows to noninvasively visualize coronary artery disease, challenging the role of stress testing as the default noninvasive imaging tool for evaluating patients with chest pain. In this review, we summarize current data on the clinical utility of cardiac computed tomography and stress testing in stable patients with suspected coronary artery disease.
Keywords: Coronary heart disease, cardiac computed tomography angiography, stress imaging, myocardial perfusion imaging, single-photon-emission tomography
Introduction
Cardiovascular diseases, and particular, coronary artery disease (CAD), remain the leading cause of death worldwide with an enormous burden on health care systems [1]. Annually, more than 10 million stress tests and approximately one million diagnostic cardiac catheterizations are being performed in the U.S. alone [1]. Total costs of cardiovascular disease and stroke in the U.S. for 2015 are estimated to exceed 320 billion dollars [1]. Management of CAD requires an accurate diagnosis. For many decades, invasive coronary angiography (ICA) has served as the gold standard for the diagnosis of CAD despite many well recognized limitations of this seasoned technology [2;3]. To avoid risks from cardiac catheterization in low-intermediate risk patients, we have been using myocardial stress testing as gatekeeper for invasive angiography. The emergence of multi-detector computed tomography technology has allowed to noninvasively assess the presence, location, severity, and characteristics of coronary atherosclerotic disease in patients. In recent years, an abundance of clinical studies revealed data on the diagnostic and prognostic performance of cardiac computed tomography angiography (CCTA), challenging the role of stress testing as the default noninvasive test for patients presenting with non-acute chest pain. In this paper, we review current data on the clinical utility of CCTA vs. stress testing in stable patients with suspected CAD.
Stress Testing for the Diagnosis of Coronary Artery Disease
Numerous studies and meta-analyses reported accuracy of stress testing for the diagnosis of CAD as defined by the gold standard of cardiac catheterization [4–7]. Without imaging, the sensitivity of an exercise treadmill test for detecting CAD is only modest, i.e. approximately 70%, while specificity is good (75–80%) [6]. Adding myocardial imaging to standard exercise testing increases sensitivity for detecting CAD. Single-photon-emission-computed-tomography (SPECT) is the most commonly used imaging adjunct to exercise testing in the US. In meta-analyses, exercise SPECT yields sensitivity and specificity of 87% and 64% vs. 82% and 75% when combined with pharmacologic ‘stress’ [4;8]. Stress echocardiography is used less frequently than SPECT in the US. In a meta-analysis, sensitivity and specificity for stress echocardiography was 85% and 77% compared to 80% and 84% with dobutamine [4;8]. Overall, combined stress testing with imaging yields similar diagnostic performance for either SPECT or echocardiography, with sensitivity and specificity of approximately 80–90% and 70–80%, respectively [9]. Remarkably, very few data are available from multi-center analyses using independent core laboratories. In general, multi-center data provide more realistic data on diagnostic test performance and typically reveal lower accuracies compared to the less rigorous analyses by specialized, single centers [10]. Furthermore, multicenter studies commonly require prospective enrollment and reduce inflation of sensitivity through referral or verification bias [11–13]. Recently, SPECT was compared to magnetic resonance imaging (MRI) in three multicenter studies revealing only modest accuracy for detecting CAD, with area under the curve (AUC) between 0.67–0.69 [14–16]. These results from studies with strong methodology suggest that the diagnostic accuracy of stress testing reported by less well conducted single-center studies – and widely disseminated in analyses and practice guidelines – may be overestimated and may therefore not reflect clinical practice.
CCTA for the Diagnosis of Coronary Artery Disease
Diagnostic accuracy of CCTA in stable patients with suspected CAD has been tested in numerous single center studies [17–19]. In meta-analyses, diagnostic accuracy of CCTA for identifying CAD in patients yielded AUC between 0.97–0.99. Pooled sensitivity ranges between 98–99%; specificity between 82–89% [17;18]. There are also several multicenter studies that reported AUCs of 0.93–0.96 for 64-slice detector technology among patients with a different CAD risk characteristics [20–22]. The rigorously performed CORE-64 study [23] revealed an AUC of 0.93 for detecting ≥ 50%, with sensitivity of 85% and specificity of 90%. A recent meta-analysis of 89 studies – including single and multicenter studies – reported a mean sensitivity of 0.97 (95% CI 0.96–0.98) and mean specificity of 0.87 (0.85–0.90) for detecting obstructive CAD [24]. The diagnostic accuracy data for CCTA have been largely consistent among studies when patient characteristics and methodology were considered [20]. In general, single center results yield somewhat greater accuracy compared to multicenter studies due to the aforementioned reasons. In addition, sensitivity and specificity vary slightly from study to study because of readers specific thresholds, while predictive values may exhibit larger differences owing to their dependence on disease prevalence in the study population [20]. Even an accurate test will see decreasing negative predictive values if disease prevalence is high [20]. Furthermore, severe coronary calcification leads to decreased diagnostic accuracy for CCTA because of lower specificity. Yet, overall diagnostic performance remains excellent even when including patients with high calcium scores [20].
Direct Comparison of Stress Testing to CCTA for the Diagnosis of CAD
Fewer data are available on the diagnostic performance of stress testing and CCTA in direct comparison. In a review of 7 small clinical studies, CCTA yielded a sensitivity of 96% vs. 66% by SPECT [9]. In a meta-analysis evaluating 11 studies with 1,575 patients, which also included data from 16-slice CT technology, the investigators found a pooled sensitivity for CCTA vs. exercise electrocardiography and SPECT of 98% (95% CI: 93% to 99%) vs. 67% (95% CI: 54% to 78%) (p<0.001) and 99% (95% CI: 96% to 100%) vs. 73% (95% CI: 59% to 83%), respectively. The pooled specificity of CCTA was 82% (95% CI: 63% to 93%) vs. 46% (95% CI: 30% to 64%, p<0.001) for exercise electrocardiography, and 71% (95% CI: 60% to 80%) vs. 48% (95% CI: 31% to 64%, P=0.14) for SPECT [25]. However, the retrospective study design and lack of core laboratory analysis in these studies render their results inconclusive. Recently, data were presented from the CORE320 multicenter study on a direct comparison of CCTA and SPECT revealing significantly greater accuracy for CCTA with an AUC of 0.91 vs. 0.69 [26]. Similar to the single center results from direct comparisons, sensitivity was markedly superior for CCTA (92%) compared to SPECT (62%) in this rigorously performed analysis. Furthermore, the diagnostic accuracy for SPECT was in line with data from the multicenter CE-MARC study, i.e., consistent with studies using meticulous methodology, but deviant from results obtained in specialized SPECT centers without core laboratory analysis. In support of the findings from multicenter studies, the CATCH study reported a positive predictive value for CCTA to diagnose CAD of 71% vs. only 36% for standard care using stress testing [27]. In the SCOT-HEART multicenter study [28], 9,849 patients with stable chest pain were randomized to two groups, CCTA plus standard of care and standard of care alone, and were followed for a mean of 1.7 years. At 6 weeks, CCTA resulted in reclassification of CAD diagnosis in 558 (27%) patients vs. 22 (1%) in standard of care group (p<0.0001). Furthermore, the greater number of patients identified with CAD led to changes in preventative measures and to a 38% reduction in fatal and non-fatal myocardial infarctions (p=0.0527). Table 1 summarizes the diagnostic accuracy of CCTA vs. stress testing.
Table 1.
Direct Comparison of Diagnostic Accuracy by CCTA and Stress Testing for the Diagnosis of Coronary Artery Disease in Patients
| Test | N | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| CCTA SPECT, stenosis>50% [26] |
381 | 91% 62% |
74% 67% |
83% 73% |
85% 55% |
| CCTA SPECT, stenosis>70% [26] |
381 | 94% 71% |
60% 67% |
55% 73% |
82% 73% |
| CCTA SPECT, stenosis>50% [83] |
122 | 98.9% 56% |
74.2% 38.7% |
91.8% 72.9% |
95.8% 23% |
| CCTA SPECT, stenosis>70% [83] |
122 | 89.7% 57.7% |
86.4% 43.2% |
92.1% 64.3% |
82.6% 36.5% |
| CCTA SPECT [84] |
58 | 100% 59% |
81% 48% |
NA | NA |
| CCTA SPECT [85] |
47 | 100% 69% |
73% 36% |
92% 78% |
100% 27% |
| CCTA SPECT [86] |
254 | 97.1% 81.8% |
54.2% 32.4% |
90.3% 74.2% |
81.3% 42.9% |
| CCTA XECG [87] |
62 | 100% 78% |
87% 67% |
96% 89% |
100% 47% |
| CCTA XECG [88] |
80 | 91% 73% |
83% 31% |
98% 67% |
91% 65% |
| CCTA XECG [89] |
98 | 96% 71% |
37% 76% |
67% 80% |
88% 66% |
| CCTA XECG [90] |
100 | 96% 71% |
84% 38% |
70% 32% |
98% 77% |
| CCTA XECG [91] |
177 | 100% 46.2% |
98.7% 16.6% |
92.9% 8.7% |
100% 64.1% |
CCTA: Cardiac Computed Tomography Angiography; CAD: Coronary Artery Disease; XECG: exercise electrocardiography.
Prognostic Value of Stress Testing
There is abundant information on patient prognosis associated with stress test results. Most data derive from the nuclear medicine literature. In a meta-analysis with an average follow-up period of 22 and 20 months for pharmacological and exercise stress tests, respectively, the annualized rate of myocardial infarction and cardiac death was 0.65% for patients who had a normal exercise SPECT but 1.78% for a normal pharmacologic SPECT [29]. Conversely, adverse event rates were 4.30% in patients with abnormal exercise SPECT and 9.98% with abnormal pharmacologic SPECT results. In low risk patients, a negative exercise test alone – without imaging -is associated with excellent prognosis as seen in 1,461 patients with a low-risk Duke Treadmill Score [30]. Exercise capacity adds to the imaging information with stress testing [31]. SPECT results were also evaluated for risk assessment in several large clinical studies. However, all these analyses were performed post-hoc and thus, are inflicted with the associated limitations. In a COURAGE substudy, there was greater 5-year survival without myocardial infarction in patients with vs. without myocardial ischemia reduction; however there were no statistically significant differences after risk adjustment [32]. In a second COURAGE imaging substudy, the investigators found similar 7-year event rates regardless of the extent of ischemia by SPECT or treatment assignment (medical therapy alone or PCI) [33]. In the BARI 2D SPECT substudy, the percent ischemic myocardium was not significantly associated with outcome [34]. In the STICH imaging substudy, the presence of ischemia by SPECT or echocardiography did not predict outcome [35]. In the DIAD study of CAD screening using SPECT in 1,123 asymptomatic patients with type II diabetes, abnormal stress testing results were predictive of adverse events but did not improve patient outcome [36].
Prognostic Value of CCTA
Within a few years, a large body of literature has been published on the diagnostic value of CCTA. While most data report intermediate follow up times – similar to the nuclear medicine literature – a few long term studies are also available. In a large meta-analysis, the association of cardiac events with CAD evaluation by 64-slice or greater CCTA was analyzed [37]. The study consisted of more than 82,000 patient-years with over 2,000 ‘hard’ events. The analysis showed a robust association between cardiac death or MI, all-cause mortality and composite MACE, and the presence and severity of CAD by CCTA. Conversely, in 38% of patients with normal CCTA findings, annualized rate of myocardial infarction or cardiac death was only 0.04% [37]. The presence of non-obstructive disease in 1/3 of patients was associated with significantly higher cardiac death or MI, all-cause mortality and composite MACE in this group compared with patients without detected CAD by CCTA (odds ratio of 6.41 [95% CI, 2.44 to 16.84]). In addition, all-cause mortality incrementally increased with CAD severity [37]. In a study with long term follow up (mean of 80 months) in 4,244 symptomatic patients who underwent CCTA, mortality was 4.2% vs. 10.8% in 1706 patients who underwent standard of care [38]. In a study assessing long term follow up after a normal CCTA, the annual rate of major adverse events was 0.9% [39]. The CONFIRM registry followed 24,775 patients who underwent ≥64-detector row CCTA for a median of 2.3 years for the occurrence of death [40]. While a normal CCTA was associated with 0.28% annualized mortality rate, hazard ratios progressively increased for nonobstructive CAD, obstructive 1-vessel, 2-vessel, 3-vessel or left main disease with 1.62 (95% CI: 1.20 to 2.19; p=0.002), 2.00 (95% CI: 1.43 to 2.82; p<0.0001), 2.92 (95% CI: 2.00 to 4.25; p<0.0001), 3.70 (95% CI: 2.58 to 5.29; p<0.0001) respectively.
CCTA versus Stress Testing for the Prognosis of CAD
Only few studies directly compared CCTA vs. stress testing for CAD prognosis. In a study of 541 patients who underwent both CCTA and SPECT and were followed for a median of 672 days, CCTA analysis resulted in incremental prognostic value over SPECT for total mortality. The annualized mortality rate was 1.1 % in patients with normal SPECT results compared to 0.3% in patients with normal CCTA [41]. In another study, outcome of patients after CCTA (n=693) was compared to that after SPECT (n=3,067) using a matched cohort comparison study design, reporting similar annual mortality rates for CCTA (1.16%) and SPECT (1.13%) [42]. In the SPARC study [43], the 2-year-event rate for nonfatal myocardial infarction and death was 0.7% for CCTA, 1.6% for SPECT, and 5.5% for positron emission tomography (PET) in 1,703 patients with suspected CAD. In a study by Shreibati et al. in 8,820 patients undergoing CCTA and 132,343 patients with stress testing, CCTA was associated with a 40% reduction in acute myocardial infarction rates after 6 months follow up [44]. Seven non-randomized studies comprising 216,603 patients with a mean follow-up period of 20 months revealed an odds ratio for myocardial infarction of 0.53 (95% CI, 0.39–0.72, P< 0.001) for CCTA vs. exercise ECG/SPECT testing [45]. Table 2 summarizes the prognostic accuracy of CCTA vs. stress testing in different studies.
Table 2.
Annualized Rates of Myocardial Infarction and Cardiac Death According to CCTA and Stress Testing Imaging Results
| Test | CCTA [37] | Exercise treadmill test [92] | Exercise Nuclear MPI [29] | Pharmacologic Nuclear MPI [29] | Exercise Echocardiography [93] | Dobutamine Echocardiography [94] |
|---|---|---|---|---|---|---|
| N | 41,960 | 1,647 | 9,930 | 4,988 | 4,347 | 1,930 |
| Median Follow-up (months) | 23 | 30 | 20 | 22 | 36 | 32 |
| MI/Cardiac death with normal test | 0.02% | 0.80% | 0.65% | 1.78% | 0.5% | 1.13% |
| MI/Cardiac death with abnormal test | 3.38% | 2.00% | 4.30% | 9.98% | 2.06% | 4.33% |
CCTA; Cardiac Computed Tomography Angiography, CAD; Coronary Artery Disease, MPI; Myocardial perfusion imaging.
Patients Management Based on Stress test and CCTA results
Available data suggest for CCTA to yield greater diagnostic and prognostic information in patients with suspected CAD compared to stress testing but it remains unclear if such information translates into improved patient outcome. Intuitively, detection of non-obstructive CAD by CCTA is an important advantage over stress testing as it may trigger appropriate preventative measures and associated reduced rates of adverse advents [46]. However, because of the relatively low event rates in this population, surveillance of many patient years is required to demonstrate such effect. Indeed, several studies showed trends to lower myocardial infarction rates with CCTA guidance of management compared to the traditional approach with stress testing. In the SPARC study, the use of aspirin and lipid-lowering agents was higher following normal/non-obstructive CCTA findings when compared with SPECT [47]; similar results were found in other studies for use of aspirin and lipid-lowering medication [48–50]. In the SCOT-HEART study, myocardial infarction rates were strongly trending lower with the CCTA guided management compared to the traditional approach using stress testing [28]. The recently completed PROMISE trial [51] randomized 10,003 patients with stable chest pain to either a management strategy using CCTA or functional tests. Patients were of low-intermediate pretest probability for obstructive CAD. The investigators found similar, low event rates after 25 months of follow-up in both groups (3.3% and 3% for CCTA and functional testing group respectively) but significantly lower rates of myocardial infarction and cardiac death in the CCTA group at 12 months (hazard ratio, 0.66; 95% CI, 0.44 to 1.00; p=0.049).
Cost Considerations
Several studies indicated greater downstream utilization of resources after CCTA use compared to traditional approaches. This may not be surprising since CCTA is more sensitive for detecting CAD than stress testing. A more sensitive test will diagnose a greater number of patients with disease who in turn will require more resources. On the other hand, CCTA also yields lower false positive rates than stress testing which may lower the rate of unnecessary referrals for cardiac catheterization. The relevant questions are if 1) the test utilization is appropriate and 2) if the test utilization is associated with better outcome. The latter typically requires longer follow up which is rarely given in contemporary studies. In an interesting analysis, Neilson et al. investigated the impact of frontline exercise stress testing vs. CCTA on downstream test utilization in low to intermediate risk symptomatic patients [52]. The authors found lower rates of downstream testing in the CCTA group compared with stress testing with fewer uses of cardiac catheterization. In a large retrospective cohort study for the diagnosis of CAD [48], economic outcome of CCTA (1,938 patients) was compared with stress testing (matched 7,752 patients). The results suggested adjusted total healthcare and CAD expenditures to be 27% and 33% lower, respectively, for patients who had CCTA compared with patients who had stress testing. In another retrospective observational study [53], one year costs for CAD management and clinical outcomes in individuals without known CAD who had CCTA (1,647 patients) were compared with matched patients (6,588 patients) who had stress testing. CCTA testing was associated with lower healthcare costs by 25.9% and lower probability of downstream testing and revascularization compared with stress testing. In another study, health care costs were lower in CCTA group (n=1,647) compared to SPECT (n=6,588), with similar rates of myocardial infarction and CAD-related hospitalization [53].
Overall, an approach of initial CCTA testing in patients with suspected CAD appears to be cost effective if it is chosen for the right patients, i.e., of intermediate pretest probability of CAD. Hulten et al. [54] estimated 15–23% cost savings with the use CCTA vs. routine care for the overall population but found that CCTA cost exceeded that of routine care if the prevalence of obstructive CAD is greater than 28%. Shreibati et al. [55] performed a retrospective, observational cohort study using claims data from a 20% random sample of 2005–2008 Medicare fee-for-service beneficiaries aged >65 years with no claims for CAD in the preceding year, who received non-emergent, noninvasive testing for CAD (n=282,830) and compared CCTA with stress testing. CCTA was associated with higher likelihood of cardiac interventions and total healthcare spending. On the other hand, there was a trend towards lower adverse event rates with the CCTA strategy. Another recent study evaluated the two-year costs in patients undergoing CCTA, PET and SPECT reporting lower costs for SPECT imaging than CCTA or PET, primarily because of fewer subsequent invasive procedures [43].
New Technologic Developments
There have been significant advancements in SPECT and CT technology in the last few years. Improvements for SPECT included better software and image reconstruction algorithms, e.g., iterative reconstruction, resolution recovery, and noise compensation algorithms, leading to lower radiation doses and better image quality [56]. Introduction of a new generation of gamma camera systems, which utilizes semiconductor detectors of cadmium zinc telluride (CZT) [57], has led to faster acquisition time, lower radiation dose (about 1 mSv for a single injection) with preserved or higher image quality compared to conventional myocardial perfusion imaging by SPECT [58;59]. Limited experience exists at this time in regards to the clinical experience with these improvements as these technologies are not widely available. Attenuation correction algorithms, on the other hand, are becoming increasingly prevalent at major medical centers. Several studies demonstrated improved specificity for the diagnosis of CAD in patients using attenuation correction, while sensitivity is similar to uncorrected SPECT [60; 61].
For CCTA, the last few years saw large reductions in radiation dose, particularly, due to prospective scan triggering and iterative reconstruction algorithms, which may lead to submillisievert doses while maintaining acceptable image quality [62]. CT innovations that are at the brink of clinical use include CT myocardial stress perfusion imaging (CTP), CT derived Fractional Flow Reserve (FFR), and transluminal attenuation gradient (TAG) CT analysis. The combination of CCTA and CTP has increased diagnostic accuracy of CCTA for the diagnosis of hemodynamically significant coronary stenosis [63–65]. CT myocardial stress perfusion imaging has recently been shown to be more accurate than SPECT for the diagnosis of CAD [66]. Combining CTA and CTP yielded and AUC of 0.87 for predicting CAD by conventional angiography which was associated with a corresponding myocardial perfusion defect by SPECT [67]. A different approach is the use of computational flow dynamics analysis of conventional CCTA data to predict pressure gradients (FFR) in the coronary arteries [68]. The application of CT-FFR has been evaluated in several studies demonstrating good diagnostic performance compared to invasively derived FFR [69–71]. However, the long and remote analysis required for CT-FFR currently presents an obstacle to clinical use. Another promising concept to derive hemodynamic information using CCTA uses transluminal attenuation gradients (TAG) to estimate blood flow restriction [72]. Preliminary data suggest improvement in diagnostic performance using TAG for detecting hemodynamically significant CAD over standard CCTA [73]. Overall, promising data exist for both stress testing and CCTA to further improved diagnostic performance and to potentially further lower radiation doses.
Discussion and Recommendations
Indirect and direct comparisons between CCTA and stress testing consistently revealed superior diagnostic performance by CCTA for the diagnosis of CAD as defined by standard definitions. The difference in diagnostic performance is particularly apparent for sensitivity while it is less pronounced for specificity. Both negative and positive predictive values are better for CCTA compared to stress testing. The results are not surprising as CCTA – like the reference standard by cardiac catheterization – is a test evaluating the coronary anatomy while stress testing assesses myocardial perfusion. Given the recent emphasis on hemodynamically significant as opposed to merely obstructive CAD, some argue for FFR as the more appropriate reference standard [74]. However, current practice guidelines in Europe and US continue to define CAD according to anatomic criteria [75–77]. This is reasonable because there is a large body of evidence demonstrating high risk of adverse events in patients with obstructive CAD – regardless if the disease is associated with hemodynamic alterations or not [78;79]. Increasing the threshold for the diagnosis of CAD to including hemodynamically significant CAD, e.g., using FFR, would leave many patients with obstructive CAD without a diagnosis of CAD and thus, without current endorsement for important preventative measures. Recent data from several large clinical series demonstrate substantial adverse event rates in patients with nonobstructive CAD detected by conventional angiography or CCTA [46;80–82]. These studies confirm the importance of detecting coronary atherosclerotic disease even when nonobstructive or hemodynamically insignificant. Therefore, we believe the diagnosis of CAD should remain linked to lumen obstruction and not to its hemodynamic consequence. Confirmation of hemodynamic significance is important, however, when considering PCI in coronary artery lesions for symptom control as no benefit – and possible harm – occurs with coronary intervention in non-flow-limiting lesions [71].
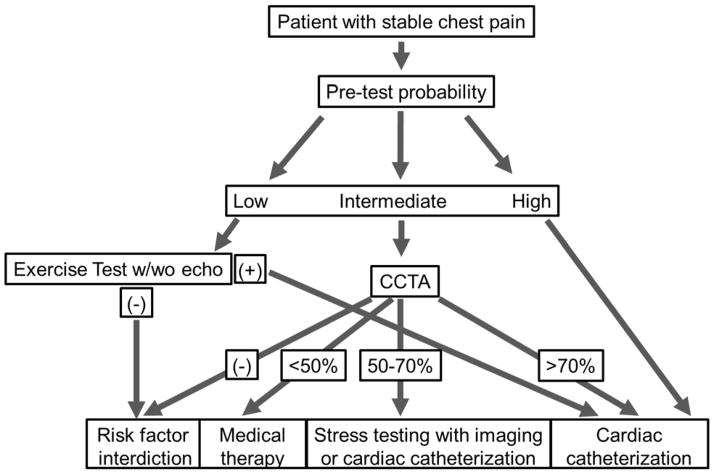
In regards to prognostic information and risk assessment, there is less clarity from head-to-head comparisons for CCTA and stress testing. However, it must be noted that the negative likelihood ratio for cardiac death and myocardial infarction associated with normal CCTA results is remarkably low and likely unmatched by stress testing when reviewing historical data. This again is expected given the inability of stress testing to detect nonobstructive disease which is associated with adverse outcome. Such advantage for risk assessment by CCTA has shown promising trends of improved patient outcome in the SCOT-HEART, PROMISE studies and in the large CONFIRM registry despite the lack of specific treatment prescriptions - a key limitation. We are still learning to optimally use the information by cardiac imaging and we currently likely to over-test and under-treat patients. For example, most patients with evidence of CAD on CCTA have low risk anatomy and require preventative measures along with medical therapy but are unlikely to benefit from cardiac catheterization/coronary artery revascularization. We may reduce risk to patients and lower cost by reserving invasive angiography for high risk patients. Future studies need to more strongly consider specific management algorithms as opposed to randomizing patients to testing without further clinical guidance. Until such data are available, we are left with our best judgment of the available data to make decisions on patient management. As always, patient characteristics and preferences are critically important to individualize decisions. Furthermore, the decision to proceed with stress testing or CCTA in patients with suspected CAD should consider the performance of the available imaging laboratories. In general terms, we believe a patient presenting with stable chest pain to a physician office will be best served with a CCTA if he is of intermediate pretest probability for CAD and if the CT laboratory has a track record of high quality scan acquisitions using low radiation dose protocols along with excellent interpretation skills. Our recommendation is particularly based on the greater sensitivity of CCTA to detect CAD compared to stress testing and based on the opportunity to implement (or withhold) preventative measures depending on the presence and extent of atherosclerotic disease. On the other hand, it appears reasonable to proceed with an exercise stress test with or without echocardiography in low risk patients as they are very unlikely to have CAD and even the risk of low level of radiation by CCTA may not be outweighed by benefit in these patients. Examples of such patients may be women under the age of 40 presenting with atypical chest pain and no risk factors for CAD. In patients with high pretest probability, stress testing with imaging or direct referral to cardiac catheterization remains a reasonable option at this time pending further data.
Figure 1.
A proposed algorithm for diagnosis of CAD in patients presenting with stable chest pain.
Abbreviations: CAD; coronary artery disease, CCTA; Cardiac Computed Tomography Angiography.
Abbreviations
- ACS
Acute Coronary Syndrome
- AUC
Area under the Curve
- CAC
coronary artery calcium
- CAD
Coronary Artery Disease
- CCTA
Cardiac Computed Tomography Angiography
- ECG
Electrocardiogram
- ICA
Invasive Coronary Angiography
- MACE
Major adverse cardiac event
- MDCT
Multi-Detector Computed Tomography
- MI
Myocardial Infarction
- MPI
Myocardial Perfusion Imaging
- NPV
Negative predictive value
- PCI
Percutaneous intervention
- PPV
Positive Predicative value
- RCT
Randomized Clinical Trial
- SPECT
Single-Photon Emission Computerized Tomography
Footnotes
Disclosures
There are no conflicts of interest to declare.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Texter J, Ostbye KM, Kitagawa K, Brinker J, George RT, et al. Quantification of lumen stenoses with known dimensions by conventional angiography and computed tomography: implications of using conventional angiography as gold standard. Heart. 2010;96(17):1358–63. doi: 10.1136/hrt.2009.186783. [DOI] [PubMed] [Google Scholar]
- 3.Arbab-Zadeh A, Hoe J. Quantification of coronary arterial stenoses by multidetector CT angiography in comparison with conventional angiography methods, caveats, and implications. JACC Cardiovasc Imaging. 2011;4(2):191–202. doi: 10.1016/j.jcmg.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA. 1998;280(10):913–20. doi: 10.1001/jama.280.10.913. [DOI] [PubMed] [Google Scholar]
- 5.Geleijnse ML, Krenning BJ, van Dalen BM, Nemes A, Soliman OI, Bosch JG, et al. Factors affecting sensitivity and specificity of diagnostic testing: dobutamine stress echocardiography. J Am Soc Echocardiogr. 2009;22(11):1199–208. doi: 10.1016/j.echo.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Gianrossi R, Detrano R, Mulvihill D, Lehmann K, Dubach P, Colombo A, et al. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation. 1989;80(1):87–98. doi: 10.1161/01.cir.80.1.87. [DOI] [PubMed] [Google Scholar]
- 7.Underwood SR, Anagnostopoulos C, Cerqueira M, Ell PJ, Flint EJ, Harbinson M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging. 2004;31(2):261–91. doi: 10.1007/s00259-003-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C, Kwok YS, Heagerty P, Redberg R. Pharmacologic stress testing for coronary disease diagnosis: A meta-analysis. Am Heart J. 2001;142(6):934–44. doi: 10.1067/mhj.2001.119761. [DOI] [PubMed] [Google Scholar]
- 9.Arbab-Zadeh A. Stress testing and non-invasive coronary angiography in patients with suspected coronary artery disease: time for a new paradigm. Heart Int. 2012;7(1):e2. doi: 10.4081/hi.2012.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A, Arbab-Zadeh A. Assessment of coronary heart disease by CT angiography: current and evolving applications. J Nucl Cardiol. 2012;19(4):796–806. doi: 10.1007/s12350-012-9556-3. [DOI] [PubMed] [Google Scholar]
- 11.Froelicher VF, Lehmann KG, Thomas R, Goldman S, Morrison D, Edson R, et al. The electrocardiographic exercise test in a population with reduced workup bias: diagnostic performance, computerized interpretation, and multivariable prediction. Veterans Affairs Cooperative Study in Health Services #016 (QUEXTA) Study Group. Quantitative Exercise Testing and Angiography. Ann Intern Med. 1998;128(12 Pt 1):965–74. doi: 10.7326/0003-4819-128-12_part_1-199806150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002;112(4):290–7. doi: 10.1016/s0002-9343(01)01111-1. [DOI] [PubMed] [Google Scholar]
- 13.Roger VL, Pellikka PA, Bell MR, Chow CW, Bailey KR, Seward JB. Sex and test verification bias. Impact on the diagnostic value of exercise echocardiography. Circulation. 1997;95(2):405–10. doi: 10.1161/01.cir.95.2.405. [DOI] [PubMed] [Google Scholar]
- 14.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29(4):480–9. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 15.Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34(10):775–81. doi: 10.1093/eurheartj/ehs022. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379(9814):453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paech DC, Weston AR. A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc Disord. 2011;11:32. doi: 10.1186/1471-2261-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mowatt G, Cook JA, Hillis GS, Walker S, Fraser C, Jia X, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94(11):1386–93. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 19.von Ballmoos MW, Haring B, Juillerat P, Alkadhi H. Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann Intern Med. 2011;154(6):413–20. doi: 10.7326/0003-4819-154-6-201103150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Arbab-Zadeh A, Miller JM, Rochitte CE, Dewey M, Niinuma H, Gottlieb I, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol. 2012;59(4):379–87. doi: 10.1016/j.jacc.2011.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 24.Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010;152(3):167–77. doi: 10.7326/0003-4819-152-3-201002020-00008. [DOI] [PubMed] [Google Scholar]
- 25*.Nielsen LH, Ortner N, Norgaard BL, Achenbach S, Leipsic J, Abdulla J. The diagnostic accuracy and outcomes after coronary computed tomography angiography vs. conventional functional testing in patients with stable angina pectoris: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15(9):961–71. doi: 10.1093/ehjci/jeu027. Meta-analysis comparing the sensitivity, specificity of CCTA againt functional testings, showing the superiority of CCTA for diagnosis of CAD in comparison with stress testings. [DOI] [PubMed] [Google Scholar]
- 26**.Di Carli MF, Arbab-Zadeh A, George RT, Chen MY, Kofoed KF, Dewey M, et al. Comparative Effectiveness of Myocardial Perfusion SPECT and Coronary CT Angiography for Diagnosis of Coronary Artery Disease. J Am Coll Cardiol. 2013;61(10) Very important multicenter study, directly comparing the accuracy of CCTA vs. SPECT for diagnosis of CAD, releaving the significantly greater accuracy in CCTA than SPECT. [Google Scholar]
- 27.Linde JJ, Kofoed KF, Sorgaard M, Kelbaek H, Jensen GB, Nielsen WB, et al. Cardiac computed tomography guided treatment strategy in patients with recent acute-onset chest pain: results from the randomised, controlled trial: CArdiac cT in the treatment of acute CHest pain (CATCH) Int J Cardiol. 2013;168(6):5257–62. doi: 10.1016/j.ijcard.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 28**.CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)60291-4. Large multicenter study, evaluating the application of CCTA for the diagnosis of CAD vs. standard of care. CCTA resulted in a better reclassification of CAD than standard of care. [DOI] [PubMed] [Google Scholar]
- 29.Navare SM, Mather JF, Shaw LJ, Fowler MS, Heller GV. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2004;11(5):551–61. doi: 10.1016/j.nuclcard.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 30.Poornima IG, Miller TD, Christian TF, Hodge DO, Bailey KR, Gibbons RJ. Utility of myocardial perfusion imaging in patients with low-risk treadmill scores. J Am Coll Cardiol. 2004;43(2):194–9. doi: 10.1016/j.jacc.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Fine NM, Pellikka PA, Scott CG, Gharacholou SM, McCully RB. Characteristics and outcomes of patients who achieve high workload (>/=10 metabolic equivalents) during treadmill exercise echocardiography. Mayo Clin Proc. 2013;88(12):1408–19. doi: 10.1016/j.mayocp.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 33.Shaw LJ, Weintraub WS, Maron DJ, Hartigan PM, Hachamovitch R, Min JK, et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J. 2012;164(2):243–50. doi: 10.1016/j.ahj.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Shaw LJ, Cerqueira MD, Brooks MM, Althouse AD, Sansing VV, Beller GA, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol. 2012;19(4):658–69. doi: 10.1007/s12350-012-9548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panza JA, Holly TA, Asch FM, She L, Pellikka PA, Velazquez EJ, et al. Inducible myocardial ischemia and outcomes in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2013;61(18):1860–70. doi: 10.1016/j.jacc.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Habib PJ, Green J, Butterfield RC, Kuntz GM, Murthy R, Kraemer DF, et al. Association of cardiac events with coronary artery disease detected by 64-slice or greater coronary CT angiography: a systematic review and meta-analysis. Int J Cardiol. 2013;169(2):112–20. doi: 10.1016/j.ijcard.2013.08.096. This important meta-analysis with consisting 82,000 patients and more than 2,000 hard event demonstrated the robust association between cardiac death or MI, all-cause mortality and composite MACE, and the presence and severity of CAD determined by CCTA. [DOI] [PubMed] [Google Scholar]
- 38.Budoff MJ, Liu S, Chow D, Flores F, Hsieh B, Gebow D, et al. Coronary CT angiography versus standard of care strategies to evaluate patients with potential coronary artery disease; effect on long term clinical outcomes. Atherosclerosis. 2014;237(2):494–8. doi: 10.1016/j.atherosclerosis.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 39.Al-Mallah MH, Qureshi W, Pantelic M, Nour K. Long term prognostic value of Coronary Computed Tomography Angiography in suspected coronary artery disease: a 62 month median follow-up study. Int J Cardiol. 2014;176(3):1244–6. doi: 10.1016/j.ijcard.2014.07.203. [DOI] [PubMed] [Google Scholar]
- 40.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58(8):849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 41.van Werkhoven JM, Schuijf JD, Gaemperli O, Jukema JW, Boersma E, Wijns W, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol. 2009;53(7):623–32. doi: 10.1016/j.jacc.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 42.Shaw LJ, Berman DS, Hendel RC, Borges NS, Min JK, Callister TQ. Prognosis by coronary computed tomographic angiography: matched comparison with myocardial perfusion single-photon emission computed tomography. J Cardiovasc Comput Tomogr. 2008;2(2):93–101. doi: 10.1016/j.jcct.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Hlatky MA, Shilane D, Hachamovitch R, Dicarli MF. Economic outcomes in the Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in Coronary Artery Disease registry: the SPARC Study. J Am Coll Cardiol. 2014;63(10):1002–8. doi: 10.1016/j.jacc.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 44.Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA. 2011;306(19):2128–36. doi: 10.1001/jama.2011.1652. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen LH, Ortner N, Abdulla J. Abstract 12801: Coronary Computed Tomography Angiography versus Conventional Functionally Testing in Patients with Stable Angina Pectoris - A Systematic Review and Meta-Analysis of Diagnostic Test Performance and Post-test Outcomes. Circulation. 2013;128(2013) [Google Scholar]
- 46.Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–91. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 47.Hachamovitch R, Nutter B, Hlatky MA, Shaw LJ, Ridner ML, Dorbala S, et al. Patient management after noninvasive cardiac imaging results from SPARC (Study of myocardial perfusion and coronary anatomy imaging roles in coronary artery disease) J Am Coll Cardiol. 2012;59(5):462–74. doi: 10.1016/j.jacc.2011.09.066. [DOI] [PubMed] [Google Scholar]
- 48.Min JK, Shaw LJ, Berman DS, Gilmore A, Kang N. Costs and clinical outcomes in individuals without known coronary artery disease undergoing coronary computed tomographic angiography from an analysis of Medicare category III transaction codes. Am J Cardiol. 2008;102(6):672–8. doi: 10.1016/j.amjcard.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 49.Min JK, Koduru S, Dunning AM, Cole JH, Hines JL, Greenwell D, et al. Coronary CT angiography versus myocardial perfusion imaging for near-term quality of life, cost and radiation exposure: a prospective multicenter randomized pilot trial. J Cardiovasc Comput Tomogr. 2012;6(4):274–83. doi: 10.1016/j.jcct.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen LH, Olsen J, Markenvard J, Jensen JM, Norgaard BL. Effects on costs of frontline diagnostic evaluation in patients suspected of angina: coronary computed tomography angiography vs. conventional ischaemia testing. Eur Heart J Cardiovasc Imaging. 2013;14(5):449–55. doi: 10.1093/ehjci/jes166. [DOI] [PubMed] [Google Scholar]
- 51**.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N Engl J Med. 2015 doi: 10.1056/NEJMoa1415516. Important clinical study which randomized more than 10,000 patients with suspected CAD to a strategy using CCTA vs. functional testing revealing equipoise for outcome of MACE but with trends favoring CCTA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nielsen LH, Markenvard J, Jensen JM, Mickley H, Ovrehus KA, Norgaard BL. Frontline diagnostic evaluation of patients suspected of angina by coronary computed tomography reduces downstream resource utilization when compared to conventional ischemia testing. Int J Cardiovasc Imaging. 2011;27(6):813–23. doi: 10.1007/s10554-010-9737-6. [DOI] [PubMed] [Google Scholar]
- 53.Min JK, Kang N, Shaw LJ, Devereux RB, Robinson M, Lin F, et al. Costs and clinical outcomes after coronary multidetector CT angiography in patients without known coronary artery disease: comparison to myocardial perfusion SPECT. Radiology. 2008;249(1):62–70. doi: 10.1148/radiol.2483071453. [DOI] [PubMed] [Google Scholar]
- 54.Hulten E, Goehler A, Bittencourt MS, Bamberg F, Schlett CL, Truong QA, et al. Cost and resource utilization associated with use of computed tomography to evaluate chest pain in the emergency department: the Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) study. Circ Cardiovasc Qual Outcomes. 2013;6(5):514–24. doi: 10.1161/CIRCOUTCOMES.113.000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA. 2011;306(19):2128–36. doi: 10.1001/jama.2011.1652. [DOI] [PubMed] [Google Scholar]
- 56.DePuey EG. Advances in SPECT camera software and hardware: currently available and new on the horizon. J Nucl Cardiol. 2012;19(3):551–81. doi: 10.1007/s12350-012-9544-7. [DOI] [PubMed] [Google Scholar]
- 57.Gambhir SS, Berman DS, Ziffer J, Nagler M, Sandler M, Patton J, et al. A novel high-sensitivity rapid-acquisition single-photon cardiac imaging camera. J Nucl Med. 2009;50(4):635–43. doi: 10.2967/jnumed.108.060020. [DOI] [PubMed] [Google Scholar]
- 58.Einstein AJ, Blankstein R, Andrews H, Fish M, Padgett R, Hayes SW, et al. Comparison of image quality, myocardial perfusion, and left ventricular function between standard imaging and single-injection ultra-low-dose imaging using a high-efficiency SPECT camera: the MILLISIEVERT study. J Nucl Med. 2014;55(9):1430–7. doi: 10.2967/jnumed.114.138222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oddstig J, Hedeer F, Jogi J, Carlsson M, Hindorf C, Engblom H. Reduced administered activity, reduced acquisition time, and preserved image quality for the new CZT camera. J Nucl Cardiol. 2013;20(1):38–44. doi: 10.1007/s12350-012-9634-6. [DOI] [PubMed] [Google Scholar]
- 60.Ficaro EP, Fessler JA, Shreve PD, Kritzman JN, Rose PA, Corbett JR. Simultaneous transmission/emission myocardial perfusion tomography. Diagnostic accuracy of attenuation-corrected 99mTc-sestamibi single-photon emission computed tomography. Circulation. 1996;93(3):463–73. doi: 10.1161/01.cir.93.3.463. [DOI] [PubMed] [Google Scholar]
- 61.Venero CV, Heller GV, Bateman TM, McGhie AI, Ahlberg AW, Katten D, et al. A multicenter evaluation of a new post-processing method with depth-dependent collimator resolution applied to full-time and half-time acquisitions without and with simultaneously acquired attenuation correction. J Nucl Cardiol. 2009;16(5):714–25. doi: 10.1007/s12350-009-9106-9. [DOI] [PubMed] [Google Scholar]
- 62.Stehli J, Fuchs TA, Bull S, Clerc OF, Possner M, Buechel RR, et al. Accuracy of coronary CT angiography using a submillisievert fraction of radiation exposure: comparison with invasive coronary angiography. J Am Coll Cardiol. 2014;64(8):772–80. doi: 10.1016/j.jacc.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 63.George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang HJ, Bluemke DA, et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009;2(3):174–82. doi: 10.1161/CIRCIMAGING.108.813766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.George RT, Arbab-Zadeh A, Miller JM, Vavere AL, Bengel FM, Lardo AC, et al. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ Cardiovasc Imaging. 2012;5(3):333–40. doi: 10.1161/CIRCIMAGING.111.969303. [DOI] [PubMed] [Google Scholar]
- 65.Ko BS, Cameron JD, Meredith IT, Leung M, Antonis PR, Nasis A, et al. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur Heart J. 2012;33(1):67–77. doi: 10.1093/eurheartj/ehr268. [DOI] [PubMed] [Google Scholar]
- 66.George RT, Mehra VC, Chen MY, Kitagawa K, Arbab-Zadeh A, Miller JM, et al. Myocardial CT perfusion imaging and SPECT for the diagnosis of coronary artery disease: a head-to-head comparison from the CORE320 multicenter diagnostic performance study. Radiology. 2014;272(2):407–16. doi: 10.1148/radiol.14140806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J. 2014;35(17):1120–30. doi: 10.1093/eurheartj/eht488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng. 2010;38(10):3195–209. doi: 10.1007/s10439-010-0083-6. [DOI] [PubMed] [Google Scholar]
- 69.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58(19):1989–97. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 70.Min JK, Berman DS, Budoff MJ, Jaffer FA, Leipsic J, Leon MB, et al. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J Cardiovasc Comput Tomogr. 2011;5(5):301–9. doi: 10.1016/j.jcct.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J Am Coll Cardiol. 2014;63(12):1145–55. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 72.Steigner ML, Mitsouras D, Whitmore AG, Otero HJ, Wang C, Buckley O, et al. Iodinated contrast opacification gradients in normal coronary arteries imaged with prospectively ECG-gated single heart beat 320-detector row computed tomography. Circ Cardiovasc Imaging. 2010;3(2):179–86. doi: 10.1161/CIRCIMAGING.109.854307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong DT, Ko BS, Cameron JD, Nerlekar N, Leung MC, Malaiapan Y, et al. Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: a comparison with fractional flow reserve. J Am Coll Cardiol. 2013;61(12):1271–9. doi: 10.1016/j.jacc.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 74.Tonino PA, De BB, Pijls NH, Siebert U, Ikeno F, Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 75.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 76.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130(19):1749–67. doi: 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 77.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 78.Arbab-Zadeh A. Fractional flow reserve-guided percutaneous coronary intervention is not a valid concept. Circulation. 2014;129(18):1871–8. doi: 10.1161/CIRCULATIONAHA.113.003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arbab-Zadeh A, Fuster V. The Myth of the “Vulnerable Plaque”: Transitioning From a Focus on Individual Lesions to Atherosclerotic Disease Burden for Coronary Artery Disease Risk Assessment. J Am Coll Cardiol. 2015;65(8):846–55. doi: 10.1016/j.jacc.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Chow BJ, Small G, Yam Y, Chen L, McPherson R, Achenbach S, et al. Prognostic and Therapeutic Implications of Statin and Aspirin Therapy in Individuals With Nonobstructive Coronary Artery Disease: Results From the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter Registry) Registry. Arterioscler Thromb Vasc Biol. 2015;35(4):981–9. doi: 10.1161/ATVBAHA.114.304351. This study, demostrated the importance of nonobstructive CAD for predicting mortality in a large clinical registry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81**.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754–63. doi: 10.1001/jama.2014.14681. Using a large VA database, this study demonstrates similar rates of myocardial infarction and death among patients with multivessel nonobstructive disease compared to patients with obstructive disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mushtaq S, De Araujo GP, Garcia-Garcia HM, Pontone G, Bartorelli AL, Bertella E, et al. Long-term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography-Leaman score. Circ Cardiovasc Imaging. 2015;8(2):e002332. doi: 10.1161/CIRCIMAGING.114.002332. [DOI] [PubMed] [Google Scholar]
- 83.Hamirani YS, Isma’eel H, Larijani V, Drury P, Lim W, Bevinal M, et al. The diagnostic accuracy of 64-detector cardiac computed tomography compared with stress nuclear imaging in patients undergoing invasive cardiac catheterization. J Comput Assist Tomogr. 2010;34(5):645–51. doi: 10.1097/RCT.0b013e3181e3d0b1. [DOI] [PubMed] [Google Scholar]
- 84.Schuijf JD, Wijns W, Jukema JW, Atsma DE, de RA, Lamb HJ, et al. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48(12):2508–14. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 85.Ravipati G, Aronow WS, Lai H, Shao J, DeLuca AJ, Weiss MB, et al. Comparison of sensitivity, specificity, positive predictive value, and negative predictive value of stress testing versus 64-multislice coronary computed tomography angiography in predicting obstructive coronary artery disease diagnosed by coronary angiography. Am J Cardiol. 2008;101(6):774–5. doi: 10.1016/j.amjcard.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 86.Tandon V, Hall D, Yam Y, Al-Shehri H, Chen L, Tandon K, et al. Rates of downstream invasive coronary angiography and revascularization: computed tomographic coronary angiography vs. Tc-99m single photon emission computed tomography. Eur Heart J. 2012;33(6):776–82. doi: 10.1093/eurheartj/ehr346. [DOI] [PubMed] [Google Scholar]
- 87.Mollet NR, Cademartiri F, Van MC, Meijboom B, Pugliese F, Runza G, et al. Adjunctive value of CT coronary angiography in the diagnostic work-up of patients with typical angina pectoris. Eur Heart J. 2007;28(15):1872–8. doi: 10.1093/eurheartj/ehl563. [DOI] [PubMed] [Google Scholar]
- 88.Dewey M, Dubel HP, Schink T, Baumann G, Hamm B. Head-to-head comparison of multislice computed tomography and exercise electrocardiography for diagnosis of coronary artery disease. Eur Heart J. 2007;28(20):2485–90. doi: 10.1093/eurheartj/ehl148. [DOI] [PubMed] [Google Scholar]
- 89.Nieman K, Galema T, Weustink A, Neefjes L, Moelker A, Musters P, et al. Computed tomography versus exercise electrocardiography in patients with stable chest complaints: real-world experiences from a fast-track chest pain clinic. Heart. 2009;95(20):1669–75. doi: 10.1136/hrt.2009.169441. [DOI] [PubMed] [Google Scholar]
- 90.Ovrehus KA, Jensen JK, Mickley HF, Munkholm H, Bottcher M, Botker HE, et al. Comparison of usefulness of exercise testing versus coronary computed tomographic angiography for evaluation of patients suspected of having coronary artery disease. Am J Cardiol. 2010;105(6):773–9. doi: 10.1016/j.amjcard.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 91.Maffei E, Seitun S, Martini C, Palumbo A, Tarantini G, Berti E, et al. CT coronary angiography and exercise ECG in a population with chest pain and low-to-intermediate pre-test likelihood of coronary artery disease. Heart. 2010;96(24):1973–9. doi: 10.1136/hrt.2009.191361. [DOI] [PubMed] [Google Scholar]
- 92.Peteiro J, Monserrrat L, Pineiro M, Calvino R, Vazquez JM, Marinas J, et al. Comparison of exercise echocardiography and the Duke treadmill score for risk stratification in patients with known or suspected coronary artery disease and normal resting electrocardiogram. Am Heart J. 2006;151(6):1324–10. doi: 10.1016/j.ahj.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 93.Elhendy A, Mahoney DW, Khandheria BK, Paterick TE, Burger KN, Pellikka PA. Prognostic significance of the location of wall motion abnormalities during exercise echocardiography. J Am Coll Cardiol. 2002;40(9):1623–9. doi: 10.1016/s0735-1097(02)02338-0. [DOI] [PubMed] [Google Scholar]
- 94.Bangalore S, Yao SS, Chaudhry FA. Prediction of myocardial infarction versus cardiac death by stress echocardiography. J Am Soc Echocardiogr. 2009;22(3):261–7. doi: 10.1016/j.echo.2008.12.022. [DOI] [PubMed] [Google Scholar]