Figure 1.
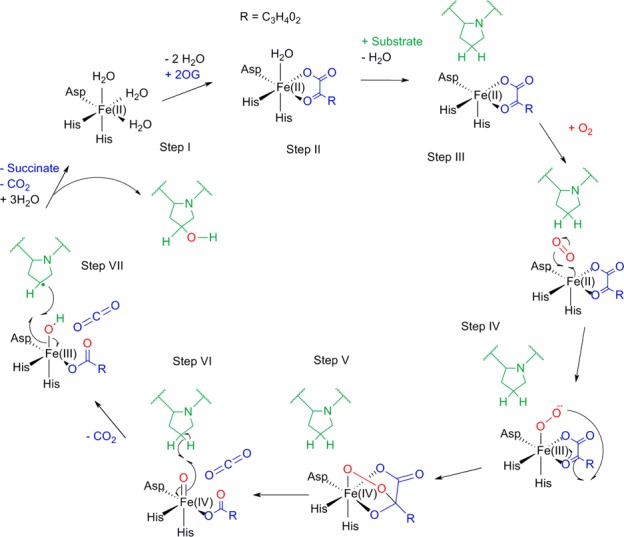
Mechanism of the Fe(II)- and 2-OG-dependent dioxygenases. In its resting position, the enzyme active site contains a ferrous iron ion coordinated by two His residues and one Asp residue (step I). The binding of 2-OG displaces two water molecules (step II); binding of a third water molecule is displaced/weakened upon substrate binding (step III). Substrate binding allows oxygen binding, which subsequently forms an anionic intermediate (step IV) that attacks the ketone of 2-OG to form a cyclic peroxide molecule (step V). The collapse of this intermediate causes formation of the Fe(IV)oxo species, which abstracts a hydrogen from the substrate (step VI). The substrate radical then reacts with the Fe(III)–OH complex to form the hydroxylated substrate (step VII) and restores the enzyme to the resting position.
